Key Insights
The global Diagnostic Tympanometers market is poised for significant expansion, projected to reach an estimated market size of approximately $650 million by 2025 and is expected to grow at a Compound Annual Growth Rate (CAGR) of around 6.5% through 2033. This robust growth is primarily driven by the increasing prevalence of hearing-related disorders, particularly in aging populations and among children, as well as a rising awareness of the importance of early diagnosis and intervention. Advancements in technology, leading to more portable, user-friendly, and sophisticated diagnostic devices, are also fueling market adoption. Furthermore, a growing emphasis on routine audiological screenings in clinical and hospital settings, coupled with expanding healthcare infrastructure in emerging economies, contributes to the positive market trajectory. The market is segmented by application into clinics, hospitals, and physical examination centers, with hospitals expected to represent a dominant share due to the higher volume of diagnostic procedures. By type, adult and children diagnostic tympanometers cater to distinct patient demographics, both experiencing growth due to specific age-related hearing health concerns.
Several key trends are shaping the Diagnostic Tympanometers landscape. The integration of artificial intelligence (AI) for enhanced data analysis and more accurate diagnosis is gaining traction. Miniaturization of devices, leading to increased portability and ease of use in various settings, including remote patient monitoring and home healthcare, is another significant trend. Moreover, the development of tympanometers with advanced features such as impedance audiometry and acoustic reflex testing, providing comprehensive middle ear assessments, is enhancing their diagnostic capabilities. Despite this promising outlook, the market faces some restraints. High initial investment costs for advanced diagnostic equipment can be a barrier for smaller healthcare facilities. Stringent regulatory approvals for medical devices can also slow down product launches and market penetration. Additionally, a shortage of trained audiologists and healthcare professionals skilled in operating these sophisticated devices in certain regions could impact market growth. However, the overall demand for accurate and efficient hearing diagnostics, coupled with continuous innovation, is expected to outweigh these challenges, ensuring sustained market expansion.
Here is a compelling, SEO-optimized report description for Diagnostic Tympanometers, integrating high-traffic keywords and market insights as requested.
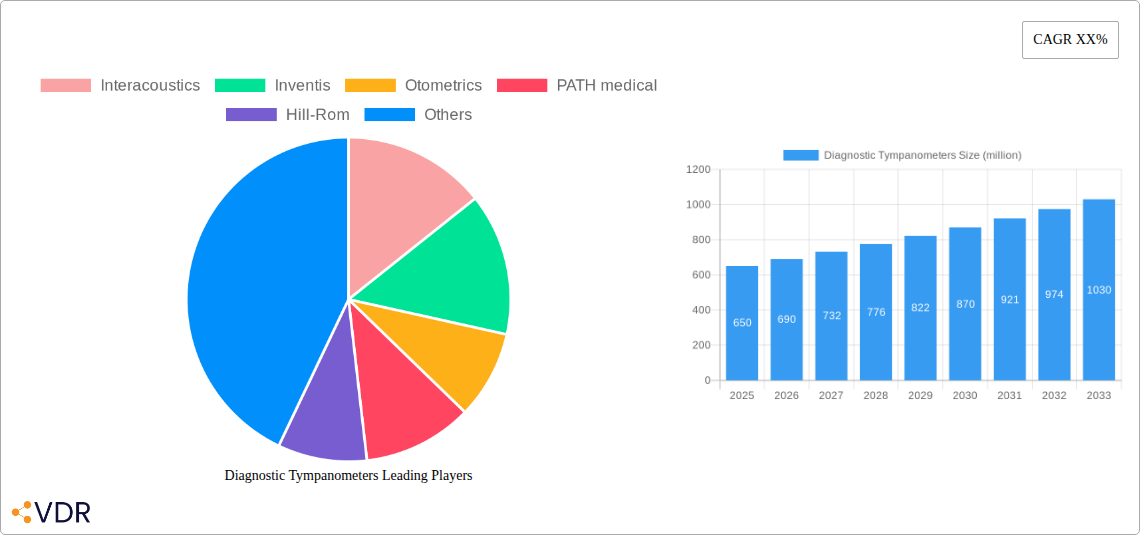
Diagnostic Tympanometers Market Dynamics & Structure
The global diagnostic tympanometers market is characterized by a moderately concentrated landscape, with key players like Interacoustics, Inventis, Otometrics, PATH medical, Hill-Rom, Grason Stadler, Maico, Oscilla, Braun International, and Audiology Systems holding significant sway. Technological innovation remains a primary driver, with advancements in impedance audiometry and real-time data analysis pushing the boundaries of diagnostic accuracy. The market is also shaped by stringent regulatory frameworks, primarily focused on medical device safety and efficacy, ensuring patient well-being. Competitive product substitutes, while not directly replacing tympanometry's core function, include broader audiological assessment tools. End-user demographics are expanding beyond traditional clinical settings to encompass telehealth platforms and point-of-care diagnostics. Merger and acquisition (M&A) trends are observed as larger entities seek to consolidate market share and expand their product portfolios, with an estimated xx M&A deals in the historical period (2019-2024). Innovation barriers include the high cost of research and development for novel sensor technologies and the lengthy regulatory approval processes.
- Market Concentration: Moderately concentrated, with a few dominant players.
- Technological Innovation: Driven by enhanced impedance measurement and digital integration.
- Regulatory Frameworks: Stringent standards ensuring device safety and clinical validity.
- End-User Demographics: Expanding from clinics to include hospitals and potentially home-use scenarios.
- M&A Trends: Strategic acquisitions aimed at portfolio expansion and market consolidation.
Diagnostic Tympanometers Growth Trends & Insights
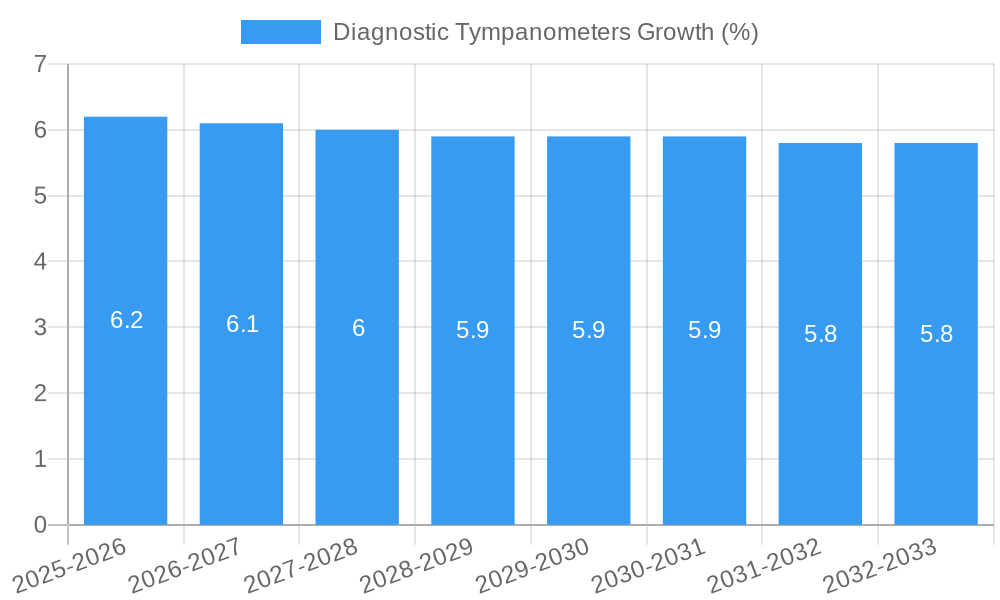
The diagnostic tympanometers market is poised for robust expansion over the forecast period of 2025–2033, exhibiting a compound annual growth rate (CAGR) of approximately xx%. This sustained growth is propelled by the increasing global prevalence of hearing impairments across all age groups and a heightened awareness of the importance of early audiological diagnosis. The market size, estimated at around $xxx million in the base year of 2025, is projected to reach an impressive $xxx million by 2033. Adoption rates are steadily climbing, particularly in developing economies where access to advanced diagnostic equipment is improving. Technological disruptions, such as the integration of artificial intelligence for data interpretation and the development of portable, user-friendly tympanometers, are revolutionizing clinical workflows and patient convenience. Consumer behavior shifts are also playing a crucial role, with a growing demand for personalized healthcare solutions and preventative audiological screenings becoming more commonplace. The increasing incorporation of diagnostic tympanometers into routine physical examinations and the rising demand for pediatric audiological assessments are significant contributing factors. The penetration of digital health platforms further amplifies the reach and utility of these devices, enabling remote monitoring and data sharing.
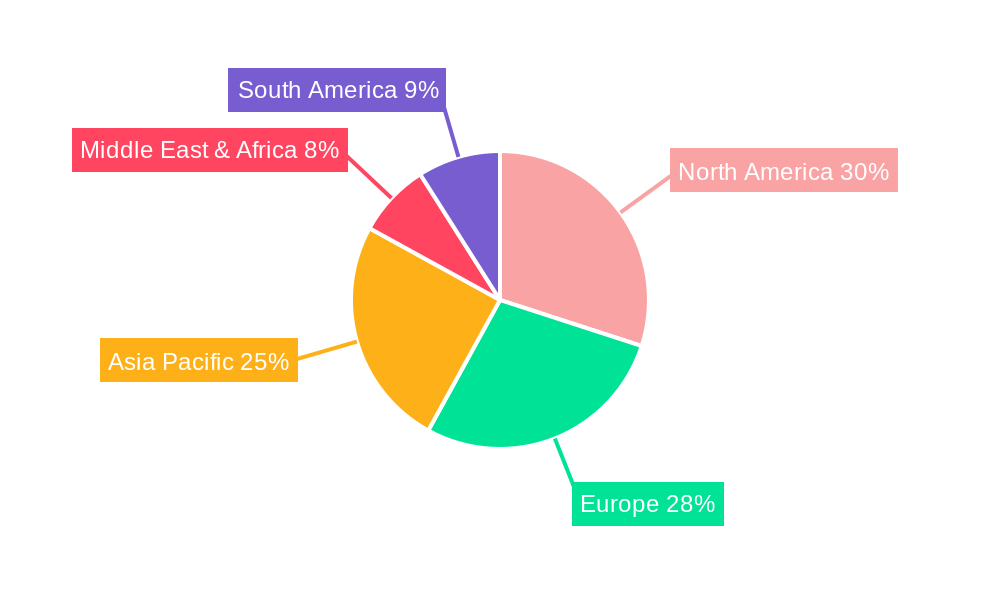
Dominant Regions, Countries, or Segments in Diagnostic Tympanometers
The Adult Diagnostic Tympanometer segment is currently the dominant force driving market growth in the diagnostic tympanometers sector, projected to maintain its lead throughout the forecast period. This dominance stems from the higher incidence of age-related hearing loss in adult populations, coupled with a greater propensity for routine health check-ups and specialized audiological evaluations. The North America region, particularly the United States, stands out as a leading country due to its advanced healthcare infrastructure, high disposable income, and proactive approach to preventive healthcare. The robust presence of major diagnostic tympanometer manufacturers and distributors further solidifies its market leadership.
- Key Drivers in Adult Diagnostic Tympanometers:
- Rising global aging population and associated hearing loss.
- Increased awareness of the impact of hearing loss on overall health and quality of life.
- Higher adoption rates in established healthcare systems for routine diagnostics.
- Integration into comprehensive audiological assessment protocols.
- Dominance Factors in North America:
- Well-established reimbursement policies for audiological services.
- Significant investment in research and development by leading companies.
- Strong network of audiologists and otolaryngologists.
- High patient demand for accurate and efficient diagnostic tools.
In the Clinic application segment, diagnostic tympanometers are experiencing peak utilization due to their indispensable role in audiology practices and general healthcare facilities. The ease of integration into existing clinical workflows and the direct patient interaction make clinics the primary hub for tympanometry. The growing emphasis on early detection of middle ear pathologies, such as otitis media, in both pediatric and adult patients further amplifies the importance of diagnostic tympanometers in clinic settings. The market share within this segment is substantial, reflecting its foundational role in audiological diagnostics.
Diagnostic Tympanometers Product Landscape
The diagnostic tympanometers market is witnessing continuous product innovation focused on enhancing diagnostic accuracy, portability, and user-friendliness. Advanced tympanometers now feature sophisticated impedance measurement algorithms, automated interpretation capabilities, and seamless integration with Electronic Health Records (EHR) systems. Innovations like real-time waveform analysis and multi-frequency impedance testing are providing deeper insights into middle ear function. The development of pediatric-specific tympanometers with child-friendly interfaces and shorter testing times is addressing a critical unmet need. Furthermore, the increasing integration of wireless connectivity and cloud-based data management solutions is streamlining clinical workflows and improving accessibility to patient data. The performance metrics of these devices are continually being refined, with a focus on reduced testing duration and improved test-retest reliability.
Key Drivers, Barriers & Challenges in Diagnostic Tympanometers
Key Drivers:
The diagnostic tympanometers market is primarily propelled by the escalating global burden of hearing loss, driven by an aging population and increased exposure to noise pollution. The growing emphasis on early detection and management of middle ear pathologies, particularly in pediatric populations, is a significant growth catalyst. Furthermore, advancements in technology, leading to more accurate, portable, and user-friendly tympanometers, are enhancing adoption rates across various healthcare settings. The expanding scope of audiological services and the increasing integration of tympanometry into routine health check-ups also contribute to market growth.
Barriers & Challenges:
Despite strong growth prospects, the market faces several challenges. The high initial cost of sophisticated diagnostic tympanometers can be a barrier to adoption, especially for smaller clinics or in resource-limited regions. Stringent regulatory approval processes can also lead to extended product development timelines and increased costs. Competitive pressures from alternative diagnostic methods, although not direct replacements, and the need for trained personnel to operate and interpret results can also pose challenges. Supply chain disruptions and the availability of skilled healthcare professionals capable of utilizing these advanced devices are also critical considerations.
Emerging Opportunities in Diagnostic Tympanometers
Emerging opportunities lie in the development of AI-powered diagnostic tympanometers that can offer automated preliminary diagnoses and risk assessments, thereby augmenting the capabilities of healthcare professionals. The expansion of telehealth and remote patient monitoring platforms presents a significant avenue for portable and connected tympanometers, allowing for audiological assessments outside traditional clinical settings. Untapped markets in developing economies, where the prevalence of hearing issues is high but access to diagnostic tools is limited, offer substantial growth potential. Innovative applications in audiology research and the development of specialized tympanometers for specific conditions, such as balance disorders, are also on the horizon.
Growth Accelerators in the Diagnostic Tympanometers Industry
Long-term growth in the diagnostic tympanometers industry will be significantly accelerated by continued technological breakthroughs, particularly in miniaturization, AI integration for predictive analytics, and enhanced sensor technologies that enable more precise measurements. Strategic partnerships between device manufacturers and leading audiology research institutions will foster innovation and accelerate the translation of new technologies into commercially viable products. Furthermore, market expansion strategies targeting underserved regions and the development of cost-effective, high-performance tympanometers will be crucial for unlocking new growth avenues and increasing market penetration.
Key Players Shaping the Diagnostic Tympanometers Market
- Interacoustics
- Inventis
- Otometrics
- PATH medical
- Hill-Rom
- Grason Stadler
- Maico
- Oscilla
- Braun International
- Audiology Systems
Notable Milestones in Diagnostic Tympanometers Sector
- 2021: Launch of advanced portable tympanometers with enhanced AI-driven interpretation features.
- 2022: Increased adoption of cloud-based data management solutions for diagnostic tympanometry.
- 2023: Introduction of specialized tympanometers designed for neonatal and infant audiological screening.
- 2024: Growing integration of diagnostic tympanometers into multi-functional audiological workstations.
- 2025: Projected increase in demand for connected tympanometers supporting remote patient monitoring.
In-Depth Diagnostic Tympanometers Market Outlook
The diagnostic tympanometers market is set for continued robust growth, fueled by an aging global population, rising awareness of hearing health, and technological advancements. Strategic imperatives will focus on expanding market penetration in emerging economies and developing innovative solutions for telehealth and personalized audiological care. The integration of AI and advanced sensor technologies will drive product differentiation and enhance diagnostic capabilities, creating a dynamic and evolving market landscape with significant opportunities for stakeholders.
Diagnostic Tympanometers Segmentation
-
1. Application
- 1.1. Clinic
- 1.2. Hospital
- 1.3. Physical Examination Center
-
2. Types
- 2.1. Adult Diagnostic Tympanometer
- 2.2. Children Diagnostic Tympanometer
Diagnostic Tympanometers Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Diagnostic Tympanometers REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Clinic
- 5.1.2. Hospital
- 5.1.3. Physical Examination Center
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Adult Diagnostic Tympanometer
- 5.2.2. Children Diagnostic Tympanometer
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Clinic
- 6.1.2. Hospital
- 6.1.3. Physical Examination Center
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Adult Diagnostic Tympanometer
- 6.2.2. Children Diagnostic Tympanometer
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Clinic
- 7.1.2. Hospital
- 7.1.3. Physical Examination Center
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Adult Diagnostic Tympanometer
- 7.2.2. Children Diagnostic Tympanometer
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Clinic
- 8.1.2. Hospital
- 8.1.3. Physical Examination Center
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Adult Diagnostic Tympanometer
- 8.2.2. Children Diagnostic Tympanometer
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Clinic
- 9.1.2. Hospital
- 9.1.3. Physical Examination Center
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Adult Diagnostic Tympanometer
- 9.2.2. Children Diagnostic Tympanometer
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Diagnostic Tympanometers Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Clinic
- 10.1.2. Hospital
- 10.1.3. Physical Examination Center
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Adult Diagnostic Tympanometer
- 10.2.2. Children Diagnostic Tympanometer
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Interacoustics
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Inventis
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Otometrics
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 PATH medical
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Hill-Rom
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Grason Stadler
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Maico
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Oscilla
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Braun International
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Audiology Systems
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Interacoustics
List of Figures
- Figure 1: Global Diagnostic Tympanometers Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Diagnostic Tympanometers Revenue (million), by Application 2024 & 2032
- Figure 3: North America Diagnostic Tympanometers Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Diagnostic Tympanometers Revenue (million), by Types 2024 & 2032
- Figure 5: North America Diagnostic Tympanometers Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Diagnostic Tympanometers Revenue (million), by Country 2024 & 2032
- Figure 7: North America Diagnostic Tympanometers Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Diagnostic Tympanometers Revenue (million), by Application 2024 & 2032
- Figure 9: South America Diagnostic Tympanometers Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Diagnostic Tympanometers Revenue (million), by Types 2024 & 2032
- Figure 11: South America Diagnostic Tympanometers Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Diagnostic Tympanometers Revenue (million), by Country 2024 & 2032
- Figure 13: South America Diagnostic Tympanometers Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Diagnostic Tympanometers Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Diagnostic Tympanometers Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Diagnostic Tympanometers Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Diagnostic Tympanometers Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Diagnostic Tympanometers Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Diagnostic Tympanometers Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Diagnostic Tympanometers Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Diagnostic Tympanometers Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Diagnostic Tympanometers Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Diagnostic Tympanometers Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Diagnostic Tympanometers Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Diagnostic Tympanometers Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Diagnostic Tympanometers Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Diagnostic Tympanometers Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Diagnostic Tympanometers Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Diagnostic Tympanometers Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Diagnostic Tympanometers Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Diagnostic Tympanometers Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Diagnostic Tympanometers Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Diagnostic Tympanometers Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Diagnostic Tympanometers Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Diagnostic Tympanometers Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Diagnostic Tympanometers Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Diagnostic Tympanometers Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Diagnostic Tympanometers Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Diagnostic Tympanometers Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Diagnostic Tympanometers Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Diagnostic Tympanometers Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Diagnostic Tympanometers?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Diagnostic Tympanometers?
Key companies in the market include Interacoustics, Inventis, Otometrics, PATH medical, Hill-Rom, Grason Stadler, Maico, Oscilla, Braun International, Audiology Systems.
3. What are the main segments of the Diagnostic Tympanometers?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4900.00, USD 7350.00, and USD 9800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Diagnostic Tympanometers," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Diagnostic Tympanometers report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Diagnostic Tympanometers?
To stay informed about further developments, trends, and reports in the Diagnostic Tympanometers, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



