Key Insights
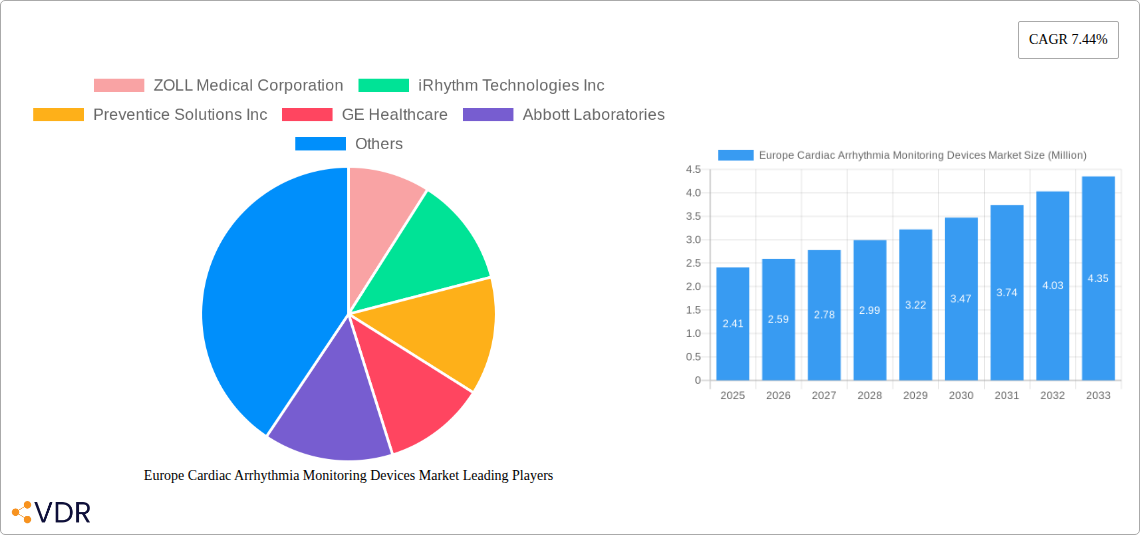
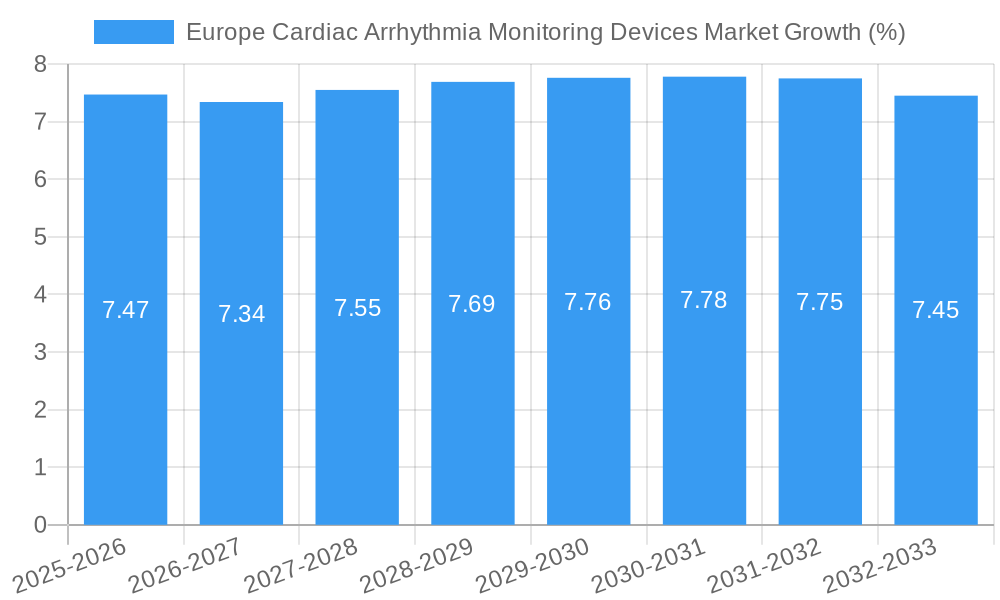
The European Cardiac Arrhythmia Monitoring Devices Market is poised for significant expansion, with a current market size of approximately USD 2.41 million, projected to grow at a robust Compound Annual Growth Rate (CAGR) of 7.44% over the forecast period of 2025-2033. This substantial growth trajectory is primarily driven by the increasing prevalence of cardiovascular diseases and arrhythmias across the continent, coupled with a heightened awareness among the population regarding the importance of early diagnosis and continuous monitoring. Technological advancements in wearable and implantable devices are further fueling market expansion, offering patients more sophisticated, non-invasive, and convenient solutions for managing their cardiac health. These innovations include the development of event monitoring devices that capture intermittent arrhythmias, continuous rhythm monitoring devices for prolonged observation, and advanced Holter monitoring devices that provide detailed, long-term electrocardiogram data. The growing demand for remote patient monitoring solutions, accelerated by the recent global health events, also plays a crucial role, enabling healthcare providers to manage patients more effectively and reduce hospital readmissions.
The market is segmented into both Devices and Service Providers, indicating a comprehensive ecosystem catering to cardiac arrhythmia management. Key device segments include Event Monitoring Devices, Continuous Rhythm Monitoring Devices, and Holter Monitoring Devices, each addressing different patient needs and clinical requirements. The Service Providers segment, encompassing Mobile Cardiac Telemetry (MCT) services and Independent Diagnostic Testing Facilities/Clinics, is essential for the effective deployment and interpretation of data generated by these devices. Prominent players such as ZOLL Medical Corporation, iRhythm Technologies Inc., Preventice Solutions Inc., GE Healthcare, Abbott Laboratories, and Medtronic PLC are actively investing in research and development to introduce innovative products and expand their market reach. The European region, with established healthcare infrastructures and a proactive approach to adopting new medical technologies, presents a fertile ground for market growth. Within Europe, countries like Germany, the United Kingdom, France, Italy, and Spain are expected to be key contributors to this expansion, supported by favorable reimbursement policies and a strong focus on preventative healthcare.
Europe Cardiac Arrhythmia Monitoring Devices Market: In-Depth Analysis and Future Outlook (2019-2033)
This comprehensive report provides an in-depth analysis of the Europe Cardiac Arrhythmia Monitoring Devices Market, offering critical insights into market dynamics, growth trends, regional dominance, product landscape, key drivers, emerging opportunities, and the competitive environment. Covering the historical period of 2019–2024 and projecting growth through 2033, with a base and estimated year of 2025, this report is an essential resource for stakeholders seeking to understand and capitalize on the evolving European market for cardiac rhythm management solutions. The report meticulously examines various market segments, including Devices (Event Monitoring Devices, Continuous Rhythm Monitoring Devices, Holter Monitoring Devices, Other Devices) and Service Providers (Mobile C, Independent Diagnostic Testing Facilities/Clinics), providing granular data and strategic perspectives.
Europe Cardiac Arrhythmia Monitoring Devices Market Market Dynamics & Structure
The Europe Cardiac Arrhythmia Monitoring Devices Market exhibits a dynamic and evolving structure, characterized by increasing technological integration and a growing demand for proactive cardiac health management. Market concentration is moderate, with key players investing heavily in research and development to introduce innovative solutions. Technological innovation is a primary driver, with advancements in wearable sensors, AI-powered diagnostics, and miniaturized implantable devices transforming patient care. Regulatory frameworks, particularly the CE Mark in Europe, play a crucial role in market access and product approvals, influencing the pace of innovation and commercialization. Competitive product substitutes are emerging, ranging from advanced wearable fitness trackers with rudimentary ECG capabilities to sophisticated implantable cardiac monitors, compelling established players to continuously enhance their offerings. End-user demographics are shifting, with an aging population and rising prevalence of cardiovascular diseases contributing to sustained market growth. Merger and acquisition (M&A) trends are observed as companies seek to expand their product portfolios, gain market share, and acquire innovative technologies. For instance, numerous strategic partnerships and acquisitions have occurred within the last five years, aiming to consolidate market presence and foster synergistic growth. The market is projected to see a steady increase in M&A deal volumes as companies look to strengthen their competitive positions. Barriers to innovation include high development costs for advanced medical devices and stringent regulatory approval processes.
- Market Concentration: Moderate, with significant R&D investments from leading players.
- Technological Innovation: Driven by AI, miniaturization, and advanced sensor technology in wearable and implantable devices.
- Regulatory Frameworks: CE Mark approval is critical, influencing market entry and product lifecycle.
- Competitive Landscape: Intense competition from established medical device manufacturers and emerging tech companies offering novel monitoring solutions.
- End-User Demographics: Growing demand fueled by an aging European population and increasing awareness of cardiac health.
- M&A Trends: Strategic acquisitions and partnerships to enhance product portfolios and market reach.
Europe Cardiac Arrhythmia Monitoring Devices Market Growth Trends & Insights
The Europe Cardiac Arrhythmia Monitoring Devices Market is poised for significant growth, projected to expand at a Compound Annual Growth Rate (CAGR) of approximately 7.5% from 2025 to 2033. This robust expansion is underpinned by a confluence of factors, including escalating adoption rates of advanced monitoring technologies, disruptive technological advancements, and evolving consumer behavior towards proactive health management. The market size, estimated at approximately USD 1,850 Million units in 2025, is expected to reach USD 3,200 Million units by 2033. The increasing prevalence of cardiac arrhythmias across Europe, driven by lifestyle changes, an aging population, and improved diagnostic capabilities, is a fundamental growth stimulant. Furthermore, the growing demand for remote patient monitoring solutions, amplified by the digital health revolution and the COVID-19 pandemic, is accelerating the adoption of continuous rhythm and event monitoring devices. Technological disruptions, such as the integration of artificial intelligence (AI) and machine learning (ML) into diagnostic algorithms, are enhancing the accuracy and efficiency of arrhythmia detection and diagnosis. Medtronic's application of AI algorithms to LINQ II ICMs exemplifies this trend, promising to improve diagnostic insights. Patient-centric approaches, focusing on early detection and personalized treatment plans, are also gaining traction, further propelling the demand for sophisticated monitoring devices. The shift towards home-based monitoring and the increasing comfort level of patients with wearable and implantable technologies are also significant contributors to market penetration. The market penetration for advanced cardiac monitoring solutions is anticipated to rise by an average of 15% annually through the forecast period, reflecting a growing acceptance and integration into standard healthcare practices. Consumer behavior is increasingly prioritizing preventative healthcare, leading individuals to seek devices that offer continuous insights into their cardiac health, thereby contributing to the sustained growth trajectory of the market.
Dominant Regions, Countries, or Segments in Europe Cardiac Arrhythmia Monitoring Devices Market
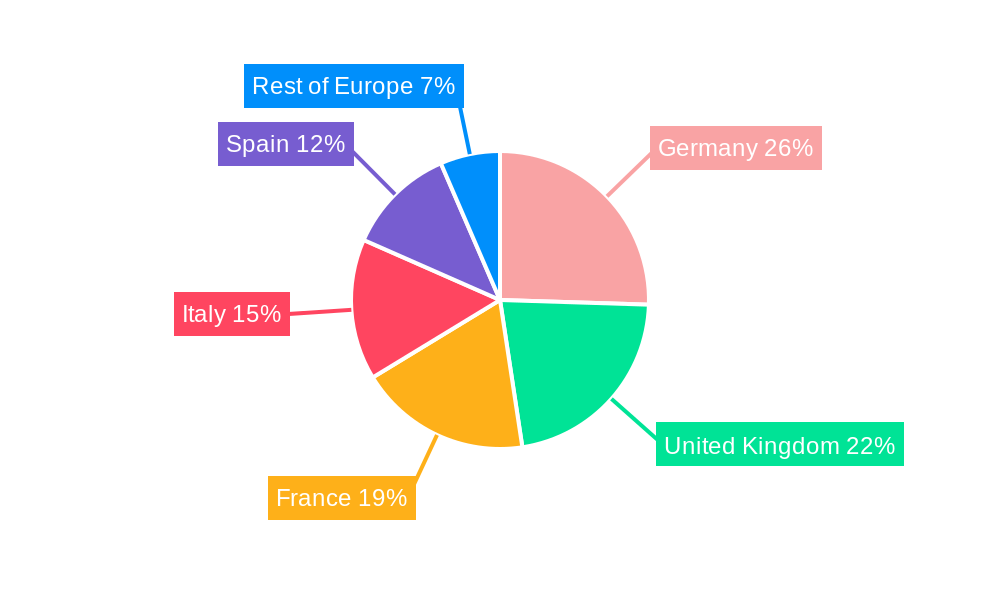
The Continuous Rhythm Monitoring Devices segment is anticipated to be the dominant force within the Europe Cardiac Arrhythmia Monitoring Devices Market, driven by their superior diagnostic capabilities and increasing adoption for long-term patient management. This segment is expected to capture a market share of approximately 40% by 2025, representing a market value of roughly USD 740 Million units. The dominance of continuous rhythm monitoring is attributed to several key drivers, including the rising incidence of paroxysmal arrhythmias that might be missed by intermittent monitoring, the growing preference for remote patient monitoring (RPM) solutions by both healthcare providers and patients, and the technological advancements that have made these devices more user-friendly and accurate. Furthermore, regulatory bodies are increasingly recognizing the value of continuous data for effective diagnosis and treatment of complex cardiac conditions.
Within this segment, Germany is projected to emerge as the leading country, accounting for an estimated 25% of the total European market share in 2025, translating to a market value of approximately USD 185 Million units. Germany's dominance stems from its robust healthcare infrastructure, high disposable income, advanced technological adoption rates, and a strong emphasis on preventative healthcare. The country boasts a high density of cardiologists and specialized cardiac centers, coupled with favorable reimbursement policies for advanced medical technologies, which significantly boost the adoption of continuous rhythm monitoring devices. Economic policies that support innovation and the integration of digital health solutions further enhance its leading position.
The Service Providers segment, specifically Independent Diagnostic Testing Facilities/Clinics, is also a crucial growth driver, catering to the increasing demand for specialized cardiac diagnostic services. These facilities play a pivotal role in managing the data generated by monitoring devices and providing expert interpretation, thereby supporting the overall market growth. Their market share is estimated to be around 30% in 2025.
Key drivers for the dominance of continuous rhythm monitoring devices and leading countries like Germany include:
- Technological Advancements: Miniaturization of devices, improved battery life, and enhanced data transmission capabilities.
- Increasing Prevalence of Arrhythmias: Growing aging population and lifestyle-related factors contributing to a higher incidence of cardiac irregularities.
- Demand for Remote Patient Monitoring: Shift towards home-based care and digital health solutions.
- Favorable Reimbursement Policies: Government and private insurance coverage for advanced monitoring technologies.
- Strong Healthcare Infrastructure: Availability of specialized cardiac centers and skilled healthcare professionals.
- Awareness and Education: Increased patient awareness regarding cardiac health and the benefits of early detection.
Europe Cardiac Arrhythmia Monitoring Devices Market Product Landscape
The product landscape of the Europe Cardiac Arrhythmia Monitoring Devices Market is characterized by continuous innovation aimed at improving diagnostic accuracy, patient comfort, and data accessibility. Key product categories include Event Monitoring Devices, Continuous Rhythm Monitoring Devices, and Holter Monitoring Devices, with emerging "Other Devices" encompassing advanced wearable solutions with integrated cardiac monitoring functionalities. Continuous Rhythm Monitoring Devices, such as insertable cardiac monitors (ICMs) and wearable continuous monitors, are at the forefront, offering prolonged data collection for diagnosing elusive arrhythmias. These devices are increasingly incorporating AI for faster and more accurate analysis of cardiac rhythm data. For example, Medtronic's LINQ II ICM, powered by AI algorithms, exemplifies this advancement, offering enhanced diagnostic capabilities. Event Monitoring Devices remain crucial for patients with infrequent symptoms, allowing them to activate recording during symptomatic episodes. Holter Monitoring Devices, while a more traditional segment, continue to be relevant for short-to-medium term monitoring. Unique selling propositions revolve around longer monitoring duration, improved signal clarity, wireless data transmission, smaller device footprints, and sophisticated algorithms for detecting a wider spectrum of arrhythmias.
Key Drivers, Barriers & Challenges in Europe Cardiac Arrhythmia Monitoring Devices Market
The Europe Cardiac Arrhythmia Monitoring Devices Market is propelled by several key drivers, including the escalating global prevalence of cardiovascular diseases and cardiac arrhythmias, a growing aging population prone to these conditions, and continuous technological advancements in diagnostic tools. The increasing adoption of remote patient monitoring (RPM) solutions and a growing emphasis on preventative healthcare also significantly contribute to market expansion. Furthermore, favorable reimbursement policies in many European countries for cardiac monitoring services and devices act as a strong growth accelerator.
However, the market also faces significant challenges and barriers. High development and manufacturing costs for sophisticated medical devices can limit affordability and accessibility, particularly in less developed European regions. Stringent regulatory approval processes and the need for extensive clinical validation can prolong time-to-market for new innovations. Supply chain disruptions, as witnessed in recent global events, can impact the availability of essential components and finished products. Moreover, data security and privacy concerns associated with transmitting sensitive patient health information are critical considerations that require robust solutions. The competitive pressure from established players and emerging companies also presents a challenge, demanding continuous innovation and strategic market positioning.
Emerging Opportunities in Europe Cardiac Arrhythmia Monitoring Devices Market
Emerging opportunities in the Europe Cardiac Arrhythmia Monitoring Devices Market lie in the burgeoning field of AI-powered diagnostics, offering enhanced predictive capabilities and personalized treatment pathways for patients. The expansion of remote patient monitoring (RPM) into post-acute care settings and the development of integrated telemedicine platforms represent significant untapped markets. Furthermore, the increasing demand for consumer-friendly wearable devices with advanced cardiac monitoring features, catering to a more proactive and health-conscious population, presents a substantial growth avenue. Opportunities also exist in developing cost-effective solutions for emerging markets within Europe and tailoring devices for specific patient populations with complex or rare arrhythmias. The integration of cardiac monitoring with other health parameters, such as blood pressure and glucose levels, to offer a holistic view of patient health, is another promising area.
Growth Accelerators in the Europe Cardiac Arrhythmia Monitoring Devices Market Industry
Long-term growth in the Europe Cardiac Arrhythmia Monitoring Devices Market is significantly driven by groundbreaking technological breakthroughs, particularly in miniaturization, sensor accuracy, and AI-driven data analysis. Strategic partnerships between medical device manufacturers, technology companies, and healthcare providers are crucial for fostering innovation and expanding market reach. The ongoing shift towards value-based healthcare models, which prioritize patient outcomes and cost-effectiveness, further accelerates the adoption of efficient and accurate monitoring solutions. Government initiatives promoting digital health adoption and telemedicine infrastructure development are also key catalysts. Market expansion strategies, including entry into underserved regions and the development of specialized monitoring solutions for niche cardiac conditions, will further fuel sustained growth.
Key Players Shaping the Europe Cardiac Arrhythmia Monitoring Devices Market Market
- ZOLL Medical Corporation
- iRhythm Technologies Inc
- Preventice Solutions Inc
- GE Healthcare
- Abbott Laboratories
- Medtronic PLC
- ACS Diagnostics Inc
- Koninklijke Philips NV
- Medi-Lynx Cardiac Monitoring LLC
- Hill-Rom Holding Inc
- Biotronik Inc
Notable Milestones in Europe Cardiac Arrhythmia Monitoring Devices Market Sector
- April 2022: LivaNova PLC received CE Mark regulatory approvals and initiated the commercial launch of the Essenz Patient Monitor in Europe. The monitoring system uses a patient-tailored approach to improve clinical efficiency and quality of patient care during Cardiopulmonary Bypass (CPB) procedures.
- April 2022: Medtronic's two AccuRhythm AI algorithms were applied to LINQ II insertable cardiac monitors (ICM) through cloud-based updates in Europe. It uses artificial intelligence to heart rhythm data collected by LINQ II, improving the accuracy of information to diagnose better and treat abnormal heart rhythms.
In-Depth Europe Cardiac Arrhythmia Monitoring Devices Market Market Outlook
- April 2022: LivaNova PLC received CE Mark regulatory approvals and initiated the commercial launch of the Essenz Patient Monitor in Europe. The monitoring system uses a patient-tailored approach to improve clinical efficiency and quality of patient care during Cardiopulmonary Bypass (CPB) procedures.
- April 2022: Medtronic's two AccuRhythm AI algorithms were applied to LINQ II insertable cardiac monitors (ICM) through cloud-based updates in Europe. It uses artificial intelligence to heart rhythm data collected by LINQ II, improving the accuracy of information to diagnose better and treat abnormal heart rhythms.
In-Depth Europe Cardiac Arrhythmia Monitoring Devices Market Market Outlook
The Europe Cardiac Arrhythmia Monitoring Devices Market is projected for sustained and robust growth, driven by an increasingly health-conscious population and the relentless pursuit of diagnostic accuracy. Future market potential lies in the seamless integration of advanced AI algorithms with implantable and wearable monitoring devices, promising predictive analytics and proactive interventions. Strategic opportunities abound in expanding remote patient monitoring services, particularly in post-operative care and for managing chronic cardiac conditions, thereby reducing hospital readmissions. The development of user-friendly, non-invasive devices that empower patients with real-time cardiac insights will also be a significant growth accelerator. Furthermore, collaborations between technology firms and healthcare providers will be instrumental in developing comprehensive digital health ecosystems that streamline data management and treatment pathways, solidifying the market's future trajectory.
Europe Cardiac Arrhythmia Monitoring Devices Market Segmentation
-
1. Devices
- 1.1. Event Monitoring Devices
- 1.2. Continuous Rhythm Monitoring Devices
- 1.3. Holter Monitoring Devices
- 1.4. Other Devices
-
2. Service Providers
- 2.1. Mobile C
- 2.2. Independent Diagnostic Testing Facilities/Clinics
Europe Cardiac Arrhythmia Monitoring Devices Market Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Cardiac Arrhythmia Monitoring Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 7.44% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Use of Telecardiology; Ease of Use and Portability of Cardiac Arrhythmia Monitoring Devices; Technological Advancements
- 3.3. Market Restrains
- 3.3.1. Lack of Awareness
- 3.4. Market Trends
- 3.4.1. Holter Monitoring Devices Segment is Expected to Hold a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Devices
- 5.1.1. Event Monitoring Devices
- 5.1.2. Continuous Rhythm Monitoring Devices
- 5.1.3. Holter Monitoring Devices
- 5.1.4. Other Devices
- 5.2. Market Analysis, Insights and Forecast - by Service Providers
- 5.2.1. Mobile C
- 5.2.2. Independent Diagnostic Testing Facilities/Clinics
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Germany
- 5.3.2. United Kingdom
- 5.3.3. France
- 5.3.4. Italy
- 5.3.5. Spain
- 5.3.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Devices
- 6. Germany Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Devices
- 6.1.1. Event Monitoring Devices
- 6.1.2. Continuous Rhythm Monitoring Devices
- 6.1.3. Holter Monitoring Devices
- 6.1.4. Other Devices
- 6.2. Market Analysis, Insights and Forecast - by Service Providers
- 6.2.1. Mobile C
- 6.2.2. Independent Diagnostic Testing Facilities/Clinics
- 6.1. Market Analysis, Insights and Forecast - by Devices
- 7. United Kingdom Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Devices
- 7.1.1. Event Monitoring Devices
- 7.1.2. Continuous Rhythm Monitoring Devices
- 7.1.3. Holter Monitoring Devices
- 7.1.4. Other Devices
- 7.2. Market Analysis, Insights and Forecast - by Service Providers
- 7.2.1. Mobile C
- 7.2.2. Independent Diagnostic Testing Facilities/Clinics
- 7.1. Market Analysis, Insights and Forecast - by Devices
- 8. France Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Devices
- 8.1.1. Event Monitoring Devices
- 8.1.2. Continuous Rhythm Monitoring Devices
- 8.1.3. Holter Monitoring Devices
- 8.1.4. Other Devices
- 8.2. Market Analysis, Insights and Forecast - by Service Providers
- 8.2.1. Mobile C
- 8.2.2. Independent Diagnostic Testing Facilities/Clinics
- 8.1. Market Analysis, Insights and Forecast - by Devices
- 9. Italy Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Devices
- 9.1.1. Event Monitoring Devices
- 9.1.2. Continuous Rhythm Monitoring Devices
- 9.1.3. Holter Monitoring Devices
- 9.1.4. Other Devices
- 9.2. Market Analysis, Insights and Forecast - by Service Providers
- 9.2.1. Mobile C
- 9.2.2. Independent Diagnostic Testing Facilities/Clinics
- 9.1. Market Analysis, Insights and Forecast - by Devices
- 10. Spain Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Devices
- 10.1.1. Event Monitoring Devices
- 10.1.2. Continuous Rhythm Monitoring Devices
- 10.1.3. Holter Monitoring Devices
- 10.1.4. Other Devices
- 10.2. Market Analysis, Insights and Forecast - by Service Providers
- 10.2.1. Mobile C
- 10.2.2. Independent Diagnostic Testing Facilities/Clinics
- 10.1. Market Analysis, Insights and Forecast - by Devices
- 11. Rest of Europe Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Devices
- 11.1.1. Event Monitoring Devices
- 11.1.2. Continuous Rhythm Monitoring Devices
- 11.1.3. Holter Monitoring Devices
- 11.1.4. Other Devices
- 11.2. Market Analysis, Insights and Forecast - by Service Providers
- 11.2.1. Mobile C
- 11.2.2. Independent Diagnostic Testing Facilities/Clinics
- 11.1. Market Analysis, Insights and Forecast - by Devices
- 12. Germany Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 13. United Kingdom Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 14. France Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 15. Italy Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 16. Spain Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 17. Rest of Europe Europe Cardiac Arrhythmia Monitoring Devices Market Analysis, Insights and Forecast, 2019-2031
- 18. Competitive Analysis
- 18.1. Market Share Analysis 2024
- 18.2. Company Profiles
- 18.2.1 ZOLL Medical Corporation
- 18.2.1.1. Overview
- 18.2.1.2. Products
- 18.2.1.3. SWOT Analysis
- 18.2.1.4. Recent Developments
- 18.2.1.5. Financials (Based on Availability)
- 18.2.2 iRhythm Technologies Inc
- 18.2.2.1. Overview
- 18.2.2.2. Products
- 18.2.2.3. SWOT Analysis
- 18.2.2.4. Recent Developments
- 18.2.2.5. Financials (Based on Availability)
- 18.2.3 Preventice Solutions Inc
- 18.2.3.1. Overview
- 18.2.3.2. Products
- 18.2.3.3. SWOT Analysis
- 18.2.3.4. Recent Developments
- 18.2.3.5. Financials (Based on Availability)
- 18.2.4 GE Healthcare
- 18.2.4.1. Overview
- 18.2.4.2. Products
- 18.2.4.3. SWOT Analysis
- 18.2.4.4. Recent Developments
- 18.2.4.5. Financials (Based on Availability)
- 18.2.5 Abbott Laboratories
- 18.2.5.1. Overview
- 18.2.5.2. Products
- 18.2.5.3. SWOT Analysis
- 18.2.5.4. Recent Developments
- 18.2.5.5. Financials (Based on Availability)
- 18.2.6 Medtronic PLC
- 18.2.6.1. Overview
- 18.2.6.2. Products
- 18.2.6.3. SWOT Analysis
- 18.2.6.4. Recent Developments
- 18.2.6.5. Financials (Based on Availability)
- 18.2.7 ACS Diagnostics Inc
- 18.2.7.1. Overview
- 18.2.7.2. Products
- 18.2.7.3. SWOT Analysis
- 18.2.7.4. Recent Developments
- 18.2.7.5. Financials (Based on Availability)
- 18.2.8 Koninklijke Philips NV
- 18.2.8.1. Overview
- 18.2.8.2. Products
- 18.2.8.3. SWOT Analysis
- 18.2.8.4. Recent Developments
- 18.2.8.5. Financials (Based on Availability)
- 18.2.9 Medi-Lynx Cardiac Monitoring LLC
- 18.2.9.1. Overview
- 18.2.9.2. Products
- 18.2.9.3. SWOT Analysis
- 18.2.9.4. Recent Developments
- 18.2.9.5. Financials (Based on Availability)
- 18.2.10 Hill-Rom Holding Inc
- 18.2.10.1. Overview
- 18.2.10.2. Products
- 18.2.10.3. SWOT Analysis
- 18.2.10.4. Recent Developments
- 18.2.10.5. Financials (Based on Availability)
- 18.2.11 Biotronik Inc
- 18.2.11.1. Overview
- 18.2.11.2. Products
- 18.2.11.3. SWOT Analysis
- 18.2.11.4. Recent Developments
- 18.2.11.5. Financials (Based on Availability)
- 18.2.1 ZOLL Medical Corporation
List of Figures
- Figure 1: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Cardiac Arrhythmia Monitoring Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Region 2019 & 2032
- Table 3: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 4: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 5: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 6: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 7: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 8: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Region 2019 & 2032
- Table 9: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 11: Germany Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Germany Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 13: United Kingdom Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: United Kingdom Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 15: France Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: France Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 17: Italy Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Italy Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 19: Spain Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Spain Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 21: Rest of Europe Europe Cardiac Arrhythmia Monitoring Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Rest of Europe Europe Cardiac Arrhythmia Monitoring Devices Market Volume (K Units) Forecast, by Application 2019 & 2032
- Table 23: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 24: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 25: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 26: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 27: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 29: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 30: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 31: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 32: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 33: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 34: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 35: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 36: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 37: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 38: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 39: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 40: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 41: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 42: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 43: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 44: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 45: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 46: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 47: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 48: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 49: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 50: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 51: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 52: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
- Table 53: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Devices 2019 & 2032
- Table 54: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Devices 2019 & 2032
- Table 55: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Service Providers 2019 & 2032
- Table 56: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Service Providers 2019 & 2032
- Table 57: Europe Cardiac Arrhythmia Monitoring Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 58: Europe Cardiac Arrhythmia Monitoring Devices Market Volume K Units Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Cardiac Arrhythmia Monitoring Devices Market?
The projected CAGR is approximately 7.44%.
2. Which companies are prominent players in the Europe Cardiac Arrhythmia Monitoring Devices Market?
Key companies in the market include ZOLL Medical Corporation, iRhythm Technologies Inc, Preventice Solutions Inc, GE Healthcare, Abbott Laboratories, Medtronic PLC, ACS Diagnostics Inc, Koninklijke Philips NV, Medi-Lynx Cardiac Monitoring LLC, Hill-Rom Holding Inc, Biotronik Inc.
3. What are the main segments of the Europe Cardiac Arrhythmia Monitoring Devices Market?
The market segments include Devices, Service Providers.
4. Can you provide details about the market size?
The market size is estimated to be USD 2.41 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Use of Telecardiology; Ease of Use and Portability of Cardiac Arrhythmia Monitoring Devices; Technological Advancements.
6. What are the notable trends driving market growth?
Holter Monitoring Devices Segment is Expected to Hold a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
Lack of Awareness.
8. Can you provide examples of recent developments in the market?
April 2022- LivaNova PLC received CE Mark regulatory approvals and initiated the commercial launch of the Essenz Patient Monitor in Europe. The monitoring system uses a patient-tailored approach to improve clinical efficiency and quality of patient care during Cardiopulmonary Bypass (CPB) procedures.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Cardiac Arrhythmia Monitoring Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Cardiac Arrhythmia Monitoring Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Cardiac Arrhythmia Monitoring Devices Market?
To stay informed about further developments, trends, and reports in the Europe Cardiac Arrhythmia Monitoring Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



