Key Insights
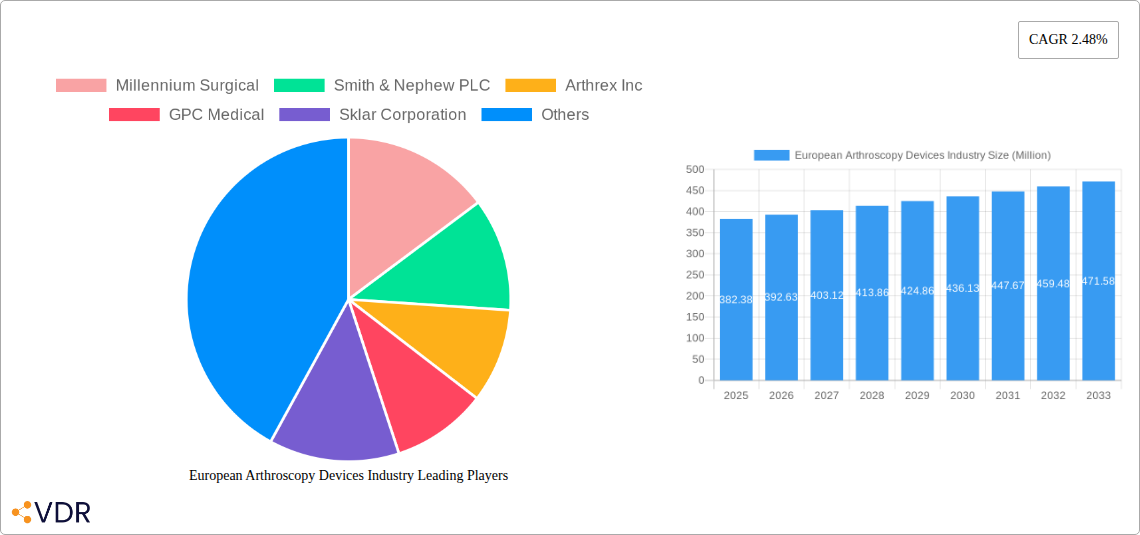
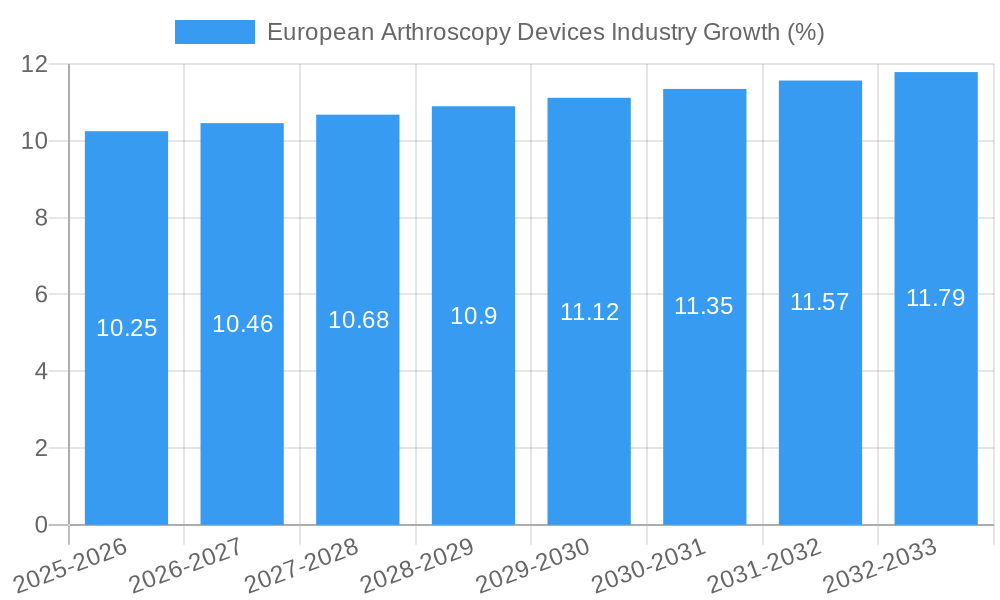
The European arthroscopy devices market, valued at €382.38 million in 2025, is projected to experience steady growth, driven by several key factors. An aging population leading to increased incidence of osteoarthritis and sports-related injuries fuels the demand for arthroscopic procedures. Technological advancements in arthroscopy equipment, such as minimally invasive instruments and improved visualization systems, enhance surgical precision and patient outcomes, further stimulating market expansion. The rising prevalence of chronic joint conditions across Europe, coupled with a growing preference for minimally invasive surgeries over traditional open surgeries, contributes significantly to market growth. Furthermore, increasing healthcare expenditure and rising insurance coverage for arthroscopic surgeries in many European countries provide a supportive environment for market expansion. However, the market might face some restraints including the high cost of advanced arthroscopy devices, which can limit accessibility in certain regions, and potential regulatory hurdles in gaining approvals for new technologies.
Specific segments within the European market show varying growth trajectories. Knee arthroscopy, as a leading application, accounts for a substantial market share driven by the high incidence of knee injuries and osteoarthritis. Hip and shoulder arthroscopies are also experiencing significant growth, reflecting the increasing prevalence of these conditions. The product segment is dominated by arthroscopes and arthroscopic implants, followed by fluid management systems and visualization systems. Germany, the UK, and France are expected to be the largest national markets within Europe, owing to their developed healthcare infrastructure and high prevalence of orthopedic conditions. While the provided data focuses on Europe, the overall market trajectory suggests a promising outlook for the foreseeable future, with a continued focus on technological innovation and minimally invasive surgical techniques.
European Arthroscopy Devices Industry Market Report: 2019-2033
This comprehensive report provides a detailed analysis of the European arthroscopy devices market, encompassing market dynamics, growth trends, competitive landscape, and future outlook. The report covers the period from 2019 to 2033, with a base year of 2025 and a forecast period of 2025-2033. The market is segmented by application (knee, hip, spine, shoulder & elbow, other) and product (arthroscope, arthroscopic implants, fluid management systems, RF systems, visualization systems, other). This in-depth analysis will equip stakeholders with crucial insights to navigate this dynamic market and make informed strategic decisions. Market values are presented in million units.
European Arthroscopy Devices Industry Market Dynamics & Structure
The European arthroscopy devices market is a moderately concentrated industry, characterized by the presence of both large multinational corporations and specialized smaller players. Technological innovation is a key driver, with continuous advancements in visualization systems, minimally invasive techniques, and implant designs leading to improved surgical outcomes and patient recovery times. Stringent regulatory frameworks, such as those set by the European Medicines Agency (EMA), influence product development and market access. The market also faces competition from alternative treatments, such as open surgery, although arthroscopy's minimally invasive nature maintains its strong position. The aging population and rising prevalence of orthopedic conditions fuel demand. Mergers and acquisitions (M&A) activity has been moderate in recent years, with larger companies seeking to expand their product portfolios and market share.
- Market Concentration: Moderately concentrated, with a Herfindahl-Hirschman Index (HHI) estimated at xx.
- Technological Innovation: Focus on advanced imaging, robotic-assisted surgery, and improved implant materials.
- Regulatory Landscape: Stringent EMA regulations impacting product approvals and market entry.
- Competitive Substitutes: Open surgery, conservative treatments.
- End-User Demographics: Aging population driving demand, increasing prevalence of osteoarthritis and sports injuries.
- M&A Activity: Moderate activity, with xx major deals recorded between 2019 and 2024.
European Arthroscopy Devices Industry Growth Trends & Insights
The European arthroscopy devices market experienced significant growth during the historical period (2019-2024), driven by factors such as increasing prevalence of musculoskeletal disorders, technological advancements, and rising healthcare expenditure. The market size is estimated at xx million units in 2025, exhibiting a Compound Annual Growth Rate (CAGR) of xx% during the historical period. Market penetration is high in major European countries, with further growth potential in underserved regions. Technological disruptions, such as the integration of robotics and artificial intelligence, are poised to transform the industry, leading to improved precision and efficiency. Consumer behavior is also shifting towards minimally invasive procedures due to shorter recovery times and reduced scarring. The forecast period (2025-2033) anticipates sustained growth, driven by similar factors and fueled by continued technological innovations.
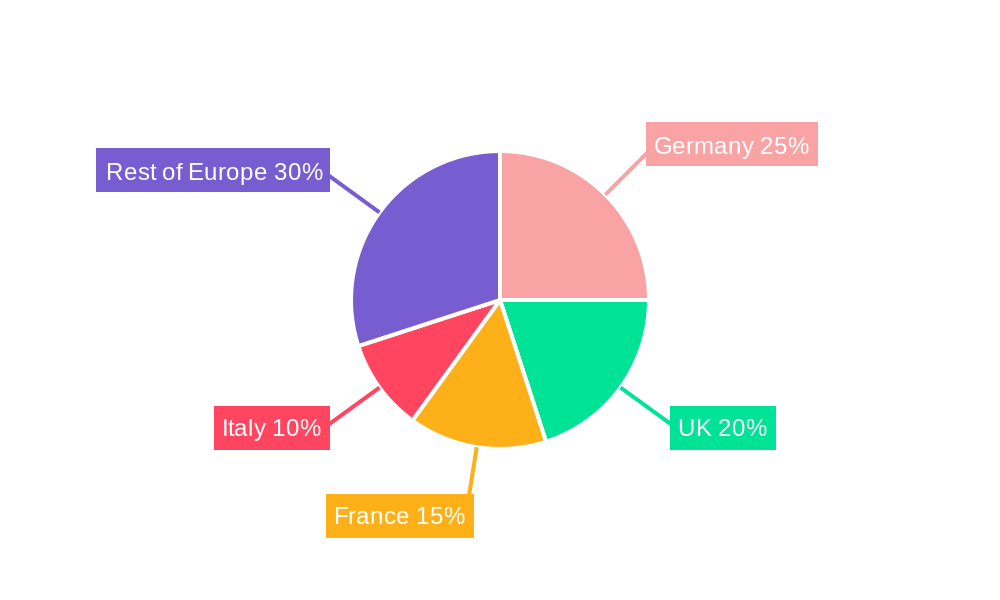
Dominant Regions, Countries, or Segments in European Arthroscopy Devices Industry
Germany, France, and the UK represent the largest markets within Europe, driven by well-established healthcare infrastructure, high prevalence of orthopedic conditions, and substantial healthcare spending. Within the application segments, knee arthroscopy dominates, representing approximately xx% of the market in 2025, followed by shoulder and elbow arthroscopy. The arthroscope segment holds the largest share within the product category, owing to its widespread use across various applications.
- Key Drivers: High healthcare expenditure, advanced healthcare infrastructure, prevalence of osteoarthritis and sports injuries.
- Germany: Largest market, benefiting from a robust healthcare system and high adoption rates.
- France: Strong market, with growing demand for minimally invasive procedures.
- UK: Significant market size, driven by a large population and increasing focus on orthopedic care.
- Knee Arthroscopy: Dominant application segment due to high prevalence of knee injuries and osteoarthritis.
- Arthroscopes: Largest product segment due to widespread use in various arthroscopic procedures.
European Arthroscopy Devices Industry Product Landscape
The European arthroscopy devices market offers a diverse range of products, including advanced arthroscopes with improved imaging capabilities, minimally invasive implants designed for optimal biocompatibility and faster healing, sophisticated fluid management systems for precise irrigation and aspiration, and radiofrequency (RF) systems for efficient tissue ablation. Visualization systems continue to evolve with enhanced resolution and 3D capabilities, while innovative designs in other products focus on improving surgical precision and ergonomics. Key selling propositions include improved surgical outcomes, reduced recovery times, and enhanced patient comfort.
Key Drivers, Barriers & Challenges in European Arthroscopy Devices Industry
Key Drivers: Technological advancements (robotics, AI), rising prevalence of musculoskeletal disorders, increasing healthcare expenditure, favorable regulatory environment (in select countries).
Key Barriers and Challenges: High cost of devices, stringent regulatory approvals, reimbursement challenges, competition from alternative treatments, potential supply chain disruptions. These factors have collectively exerted a xx% negative impact on market growth in 2024.
Emerging Opportunities in European Arthroscopy Devices Industry
Emerging opportunities lie in personalized medicine approaches, development of biocompatible and biodegradable implants, integration of advanced imaging technologies like augmented reality and artificial intelligence, and expansion into untapped markets within Eastern Europe. Moreover, increased focus on minimally invasive sports medicine procedures and the growth of ambulatory surgical centers presents further potential.
Growth Accelerators in the European Arthroscopy Devices Industry
Long-term growth will be propelled by continuous technological innovations, strategic partnerships between device manufacturers and healthcare providers, expansion into emerging markets, and increased focus on value-based healthcare models. The rise of minimally invasive surgery and increasing use of arthroscopy for sports-related injuries present substantial growth opportunities.
Key Players Shaping the European Arthroscopy Devices Industry Market
- Millennium Surgical
- Smith & Nephew PLC
- Arthrex Inc
- GPC Medical
- Sklar Corporation
- Henke Sass Wolf GmbH
- Medtronic PLC
- B Braun Melsungen AG
- Richard Wolf GmbH
- Karl Storz GmbH & Co KG
- Conmed Corporation
- Johnson & Johnson
- Stryker Corporation
- Zimmer Biomet Holdings Inc
Notable Milestones in European Arthroscopy Devices Industry Sector
- September 2022: Olympus Corporation launched VISERA ELITE III, a new surgical visualization platform with enhanced imaging capabilities, expanding its market presence in Europe and globally.
- April 2022: Smith & Nephew PLC launched the JOURNEY II Unicompartmental Knee (UK) System, featuring advanced design and customized sizing options, strengthening its position in the knee arthroscopy market.
In-Depth European Arthroscopy Devices Industry Market Outlook
The European arthroscopy devices market is poised for continued growth over the forecast period, driven by the factors outlined above. Strategic partnerships, technological advancements, and expansion into new applications and markets will be key to capturing this growth potential. Companies focusing on innovation and meeting evolving clinical needs will be best positioned to succeed in this competitive and dynamic landscape.
European Arthroscopy Devices Industry Segmentation
-
1. Application
- 1.1. Knee Arthroscopy
- 1.2. Hip Arthroscopy
- 1.3. Spine Arthroscopy
- 1.4. Shoulder and Elbow Arthroscopy
- 1.5. Other Arthroscopy Applications
-
2. Product
- 2.1. Arthroscope
- 2.2. Arthroscopic Implant
- 2.3. Fluid Management System
- 2.4. Radiofrequency (RF) System
- 2.5. Visualization System
- 2.6. Other Products
European Arthroscopy Devices Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
European Arthroscopy Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 2.48% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Incidences of Sports Injuries; Rising Geriatric Population; Technological Advancements in Arthroscopic Implants
- 3.3. Market Restrains
- 3.3.1. Lack of Skilled Surgeons; Stringent Regulatory Requirements
- 3.4. Market Trends
- 3.4.1. Knee Arthroscopy is Expected to Witness a Significant Growth Over the Forecast Period.
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Knee Arthroscopy
- 5.1.2. Hip Arthroscopy
- 5.1.3. Spine Arthroscopy
- 5.1.4. Shoulder and Elbow Arthroscopy
- 5.1.5. Other Arthroscopy Applications
- 5.2. Market Analysis, Insights and Forecast - by Product
- 5.2.1. Arthroscope
- 5.2.2. Arthroscopic Implant
- 5.2.3. Fluid Management System
- 5.2.4. Radiofrequency (RF) System
- 5.2.5. Visualization System
- 5.2.6. Other Products
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Germany
- 5.3.2. United Kingdom
- 5.3.3. France
- 5.3.4. Italy
- 5.3.5. Spain
- 5.3.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. Germany European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Knee Arthroscopy
- 6.1.2. Hip Arthroscopy
- 6.1.3. Spine Arthroscopy
- 6.1.4. Shoulder and Elbow Arthroscopy
- 6.1.5. Other Arthroscopy Applications
- 6.2. Market Analysis, Insights and Forecast - by Product
- 6.2.1. Arthroscope
- 6.2.2. Arthroscopic Implant
- 6.2.3. Fluid Management System
- 6.2.4. Radiofrequency (RF) System
- 6.2.5. Visualization System
- 6.2.6. Other Products
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. United Kingdom European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Knee Arthroscopy
- 7.1.2. Hip Arthroscopy
- 7.1.3. Spine Arthroscopy
- 7.1.4. Shoulder and Elbow Arthroscopy
- 7.1.5. Other Arthroscopy Applications
- 7.2. Market Analysis, Insights and Forecast - by Product
- 7.2.1. Arthroscope
- 7.2.2. Arthroscopic Implant
- 7.2.3. Fluid Management System
- 7.2.4. Radiofrequency (RF) System
- 7.2.5. Visualization System
- 7.2.6. Other Products
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. France European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Knee Arthroscopy
- 8.1.2. Hip Arthroscopy
- 8.1.3. Spine Arthroscopy
- 8.1.4. Shoulder and Elbow Arthroscopy
- 8.1.5. Other Arthroscopy Applications
- 8.2. Market Analysis, Insights and Forecast - by Product
- 8.2.1. Arthroscope
- 8.2.2. Arthroscopic Implant
- 8.2.3. Fluid Management System
- 8.2.4. Radiofrequency (RF) System
- 8.2.5. Visualization System
- 8.2.6. Other Products
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Italy European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Knee Arthroscopy
- 9.1.2. Hip Arthroscopy
- 9.1.3. Spine Arthroscopy
- 9.1.4. Shoulder and Elbow Arthroscopy
- 9.1.5. Other Arthroscopy Applications
- 9.2. Market Analysis, Insights and Forecast - by Product
- 9.2.1. Arthroscope
- 9.2.2. Arthroscopic Implant
- 9.2.3. Fluid Management System
- 9.2.4. Radiofrequency (RF) System
- 9.2.5. Visualization System
- 9.2.6. Other Products
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Spain European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Knee Arthroscopy
- 10.1.2. Hip Arthroscopy
- 10.1.3. Spine Arthroscopy
- 10.1.4. Shoulder and Elbow Arthroscopy
- 10.1.5. Other Arthroscopy Applications
- 10.2. Market Analysis, Insights and Forecast - by Product
- 10.2.1. Arthroscope
- 10.2.2. Arthroscopic Implant
- 10.2.3. Fluid Management System
- 10.2.4. Radiofrequency (RF) System
- 10.2.5. Visualization System
- 10.2.6. Other Products
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Rest of Europe European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Application
- 11.1.1. Knee Arthroscopy
- 11.1.2. Hip Arthroscopy
- 11.1.3. Spine Arthroscopy
- 11.1.4. Shoulder and Elbow Arthroscopy
- 11.1.5. Other Arthroscopy Applications
- 11.2. Market Analysis, Insights and Forecast - by Product
- 11.2.1. Arthroscope
- 11.2.2. Arthroscopic Implant
- 11.2.3. Fluid Management System
- 11.2.4. Radiofrequency (RF) System
- 11.2.5. Visualization System
- 11.2.6. Other Products
- 11.1. Market Analysis, Insights and Forecast - by Application
- 12. Europe European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1. undefined
- 13. Germany European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1. undefined
- 14. France European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1. undefined
- 15. United Kingdom European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1. undefined
- 16. Netherlands European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 16.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 16.1.1. undefined
- 17. Sweden European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 17.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 17.1.1. undefined
- 18. United Kingdom European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 18.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 18.1.1. undefined
- 19. Italy European Arthroscopy Devices Industry Analysis, Insights and Forecast, 2019-2031
- 19.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 19.1.1. undefined
- 20. Competitive Analysis
- 20.1. Market Share Analysis 2024
- 20.2. Company Profiles
- 20.2.1 Millennium Surgical
- 20.2.1.1. Overview
- 20.2.1.2. Products
- 20.2.1.3. SWOT Analysis
- 20.2.1.4. Recent Developments
- 20.2.1.5. Financials (Based on Availability)
- 20.2.2 Smith & Nephew PLC
- 20.2.2.1. Overview
- 20.2.2.2. Products
- 20.2.2.3. SWOT Analysis
- 20.2.2.4. Recent Developments
- 20.2.2.5. Financials (Based on Availability)
- 20.2.3 Arthrex Inc
- 20.2.3.1. Overview
- 20.2.3.2. Products
- 20.2.3.3. SWOT Analysis
- 20.2.3.4. Recent Developments
- 20.2.3.5. Financials (Based on Availability)
- 20.2.4 GPC Medical
- 20.2.4.1. Overview
- 20.2.4.2. Products
- 20.2.4.3. SWOT Analysis
- 20.2.4.4. Recent Developments
- 20.2.4.5. Financials (Based on Availability)
- 20.2.5 Sklar Corporation
- 20.2.5.1. Overview
- 20.2.5.2. Products
- 20.2.5.3. SWOT Analysis
- 20.2.5.4. Recent Developments
- 20.2.5.5. Financials (Based on Availability)
- 20.2.6 Henke Sass Wolf GmbH
- 20.2.6.1. Overview
- 20.2.6.2. Products
- 20.2.6.3. SWOT Analysis
- 20.2.6.4. Recent Developments
- 20.2.6.5. Financials (Based on Availability)
- 20.2.7 Medtronic PLC
- 20.2.7.1. Overview
- 20.2.7.2. Products
- 20.2.7.3. SWOT Analysis
- 20.2.7.4. Recent Developments
- 20.2.7.5. Financials (Based on Availability)
- 20.2.8 B Braun Melsungen AG
- 20.2.8.1. Overview
- 20.2.8.2. Products
- 20.2.8.3. SWOT Analysis
- 20.2.8.4. Recent Developments
- 20.2.8.5. Financials (Based on Availability)
- 20.2.9 Richard Wolf GmbH
- 20.2.9.1. Overview
- 20.2.9.2. Products
- 20.2.9.3. SWOT Analysis
- 20.2.9.4. Recent Developments
- 20.2.9.5. Financials (Based on Availability)
- 20.2.10 Karl Storz GmbH & Co KG
- 20.2.10.1. Overview
- 20.2.10.2. Products
- 20.2.10.3. SWOT Analysis
- 20.2.10.4. Recent Developments
- 20.2.10.5. Financials (Based on Availability)
- 20.2.11 Conmed Corporation
- 20.2.11.1. Overview
- 20.2.11.2. Products
- 20.2.11.3. SWOT Analysis
- 20.2.11.4. Recent Developments
- 20.2.11.5. Financials (Based on Availability)
- 20.2.12 Johnson & Johnson
- 20.2.12.1. Overview
- 20.2.12.2. Products
- 20.2.12.3. SWOT Analysis
- 20.2.12.4. Recent Developments
- 20.2.12.5. Financials (Based on Availability)
- 20.2.13 Stryker Corporation
- 20.2.13.1. Overview
- 20.2.13.2. Products
- 20.2.13.3. SWOT Analysis
- 20.2.13.4. Recent Developments
- 20.2.13.5. Financials (Based on Availability)
- 20.2.14 Zimmer Biomet Holdings Inc
- 20.2.14.1. Overview
- 20.2.14.2. Products
- 20.2.14.3. SWOT Analysis
- 20.2.14.4. Recent Developments
- 20.2.14.5. Financials (Based on Availability)
- 20.2.1 Millennium Surgical
List of Figures
- Figure 1: European Arthroscopy Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: European Arthroscopy Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: European Arthroscopy Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: European Arthroscopy Devices Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 4: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 5: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 6: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 7: European Arthroscopy Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 8: European Arthroscopy Devices Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 9: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 10: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 11: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 12: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 13: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 14: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 15: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 16: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 17: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 18: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 19: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 20: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 21: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 22: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 23: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 24: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 25: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 26: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 27: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 28: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 29: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 30: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 31: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 32: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 33: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 34: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 35: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 36: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 37: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 38: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 39: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 40: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 41: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 42: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 43: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 44: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 45: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 46: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 47: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 48: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 49: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 50: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 51: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 52: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 53: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 54: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 55: European Arthroscopy Devices Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 56: European Arthroscopy Devices Industry Volume K Unit Forecast, by Application 2019 & 2032
- Table 57: European Arthroscopy Devices Industry Revenue Million Forecast, by Product 2019 & 2032
- Table 58: European Arthroscopy Devices Industry Volume K Unit Forecast, by Product 2019 & 2032
- Table 59: European Arthroscopy Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 60: European Arthroscopy Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the European Arthroscopy Devices Industry?
The projected CAGR is approximately 2.48%.
2. Which companies are prominent players in the European Arthroscopy Devices Industry?
Key companies in the market include Millennium Surgical, Smith & Nephew PLC, Arthrex Inc, GPC Medical, Sklar Corporation, Henke Sass Wolf GmbH, Medtronic PLC, B Braun Melsungen AG, Richard Wolf GmbH, Karl Storz GmbH & Co KG, Conmed Corporation, Johnson & Johnson, Stryker Corporation, Zimmer Biomet Holdings Inc.
3. What are the main segments of the European Arthroscopy Devices Industry?
The market segments include Application, Product.
4. Can you provide details about the market size?
The market size is estimated to be USD 382.38 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Incidences of Sports Injuries; Rising Geriatric Population; Technological Advancements in Arthroscopic Implants.
6. What are the notable trends driving market growth?
Knee Arthroscopy is Expected to Witness a Significant Growth Over the Forecast Period..
7. Are there any restraints impacting market growth?
Lack of Skilled Surgeons; Stringent Regulatory Requirements.
8. Can you provide examples of recent developments in the market?
September 2022: Olympus Corporation launched VISERA ELITE III, the new surgical visualization platform. VISERA ELITE III offers various imaging functions. It has been launched in Europe, Japan, and many other parts of the world.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "European Arthroscopy Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the European Arthroscopy Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the European Arthroscopy Devices Industry?
To stay informed about further developments, trends, and reports in the European Arthroscopy Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



