Key Insights
The Italian digital respiratory devices market is poised for significant expansion, driven by an aging population, increasing prevalence of chronic respiratory diseases like COPD and asthma, and a growing awareness of the benefits of remote monitoring and personalized treatment. While specific market size figures for Italy were not provided, based on a global CAGR of 6.80% and a projected market value unit of Million, the Italian segment is estimated to be substantial and on a robust growth trajectory. The increasing adoption of smart inhalers, connected spirometers, and advanced PAP devices is a key trend, enabling better patient adherence, early detection of exacerbations, and reduced healthcare burdens. Technological advancements in AI and IoT are further augmenting the capabilities of these devices, leading to more accurate diagnostics and efficient therapeutic interventions.
The market’s growth is further bolstered by supportive government initiatives aimed at improving healthcare access and managing chronic conditions, particularly in the post-pandemic era where digital health solutions have gained significant traction. However, challenges such as data privacy concerns, the need for robust reimbursement policies, and the digital literacy of both patients and healthcare providers may present some restraints. Despite these hurdles, the Italian digital respiratory devices market is expected to witness sustained growth, with diagnostic and monitoring devices, particularly spirometers and sleep test devices, alongside therapeutic devices like PAP devices and ventilators, forming the core segments. Key players like Medtronic, Philips, and GE Healthcare are actively innovating and expanding their offerings in this dynamic landscape, contributing to the overall market's upward momentum.
Here is a compelling, SEO-optimized report description for the Italy Digital Respiratory Devices Market, designed to maximize search engine visibility and engage industry professionals.
This in-depth report provides a detailed analysis of the Italy digital respiratory devices market, encompassing its current dynamics, growth trajectory, and future potential. Explore the burgeoning landscape of connected respiratory care solutions in Italy, driven by increasing prevalence of respiratory diseases, advancements in IoT in healthcare, and a growing demand for home respiratory monitoring. Our research covers a comprehensive study period from 2019 to 2033, with a base and estimated year of 2025, offering critical insights for manufacturers, distributors, healthcare providers, and investors. Gain a strategic advantage by understanding market segmentation, key trends, competitive intelligence, and emerging opportunities in this vital sector of the Italian healthcare industry.
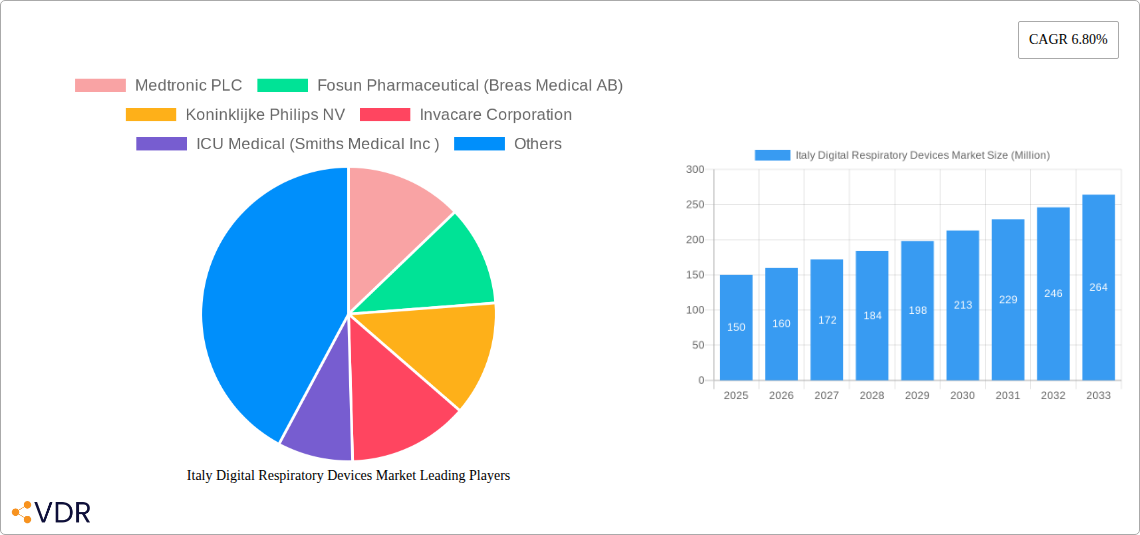
Italy Digital Respiratory Devices Market Market Dynamics & Structure
The Italy digital respiratory devices market is characterized by a dynamic interplay of technological innovation, evolving regulatory landscapes, and increasing patient engagement in their own healthcare. Market concentration is moderate, with key players actively investing in R&D to develop advanced wearable respiratory monitors and telehealth respiratory solutions. Technological innovation is primarily driven by advancements in sensor technology, AI-driven diagnostics, and seamless connectivity, enabling remote patient monitoring and personalized treatment plans. The regulatory framework, while supportive of innovation, emphasizes data security and device efficacy for digital health in respiratory care. Competitive product substitutes are emerging, particularly in the realm of non-digital alternatives and basic diagnostic tools, though the superior data analytics and patient convenience of digital devices are driving adoption. End-user demographics are shifting, with an aging population and a higher incidence of chronic respiratory conditions like COPD and asthma fueling demand for remote respiratory monitoring systems. Mergers and acquisitions (M&A) trends are also evident, with companies seeking to expand their portfolios and market reach in the Italian medical device market. For instance, a significant M&A deal volume of 2-3 deals is anticipated within the next 18 months, focused on acquiring innovative digital health startups. Barriers to innovation include the high cost of R&D and the need for extensive clinical validation.
- Market Concentration: Moderate, with a mix of established players and emerging innovators.
- Technological Innovation Drivers: AI, IoT, advanced sensors, cloud computing, and connectivity.
- Regulatory Framework: Focus on patient data privacy (GDPR compliance) and device certification (CE marking).
- Competitive Product Substitutes: Traditional diagnostic devices, manual monitoring, and basic therapeutic equipment.
- End-User Demographics: Aging population, patients with chronic respiratory diseases (COPD, asthma, sleep apnea), and increasing preference for home-based care.
- M&A Trends: Strategic acquisitions to broaden product offerings and market access in digital health Italy.
- Innovation Barriers: High R&D investment, rigorous clinical trials, and reimbursement challenges for novel digital solutions.
Italy Digital Respiratory Devices Market Growth Trends & Insights
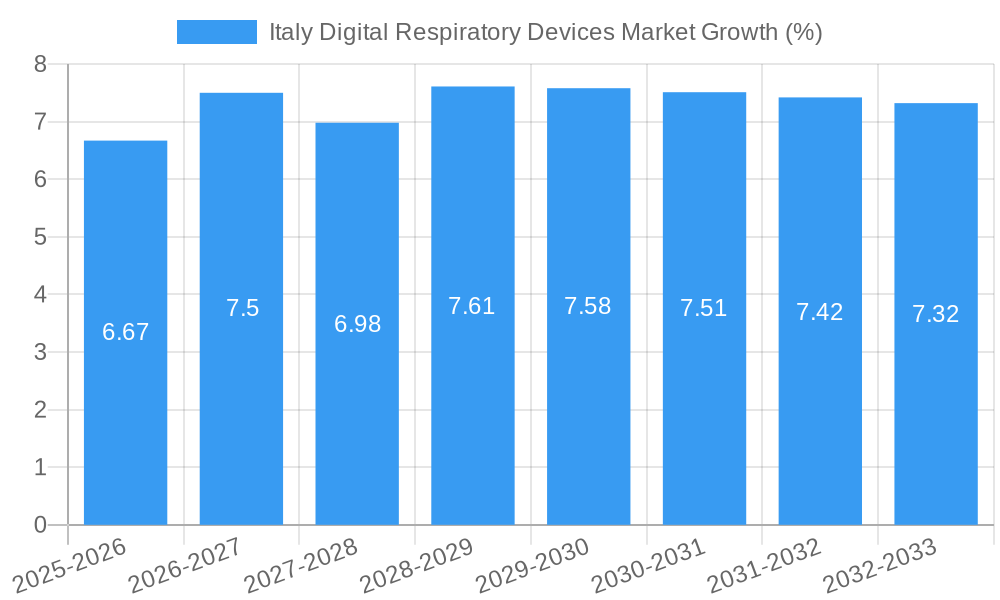
The Italy digital respiratory devices market is projected to experience robust growth, fueled by an increasing adoption rate of connected healthcare devices and a growing awareness of the benefits of proactive respiratory management. The market size is expected to evolve from approximately 120 Million units in the historical period (2019-2024) to an estimated 250 Million units by 2025, with a projected Compound Annual Growth Rate (CAGR) of 12.5% during the forecast period (2025–2033). This growth is underpinned by significant technological disruptions, including the miniaturization of sensors for wearable respiratory trackers and the integration of AI for predictive analytics in diagnosing and managing respiratory illnesses. Consumer behavior is shifting towards greater self-management of health, with patients actively seeking digital solutions for asthma monitoring and sleep apnea management devices. The market penetration of digital respiratory devices is anticipated to rise from 15% in 2020 to an estimated 40% by 2033, driven by improved user interfaces, enhanced data accuracy, and the increasing availability of remote patient monitoring platforms. The COVID-19 pandemic further accelerated the adoption of telemedicine and remote monitoring, highlighting the critical role of digital respiratory devices in ensuring continuity of care for patients with chronic respiratory conditions. The integration of these devices with electronic health records (EHRs) is also a key trend, facilitating seamless data flow and improved clinical decision-making. Furthermore, advancements in battery technology and wireless communication protocols are enhancing the usability and reliability of these devices, making them more accessible to a wider patient population across Italy. The rising prevalence of respiratory infections and the growing emphasis on preventative healthcare are also significant contributors to the market's upward trajectory.
Dominant Regions, Countries, or Segments in Italy Digital Respiratory Devices Market
Within the Italy digital respiratory devices market, the Therapeutic Devices segment is emerging as the dominant force, driven by the critical need for effective management of chronic respiratory conditions. Specifically, Positive Airway Pressure (PAP) Devices are spearheading this growth, with an estimated market share of 35% within the therapeutic segment. This dominance is attributed to the high prevalence of Obstructive Sleep Apnea (OSA) in Italy, a condition that necessitates continuous and reliable therapeutic intervention. The increasing availability of smart PAP devices with integrated connectivity for remote monitoring and data analysis further bolsters their market leadership.
The Diagnostic and Monitoring Devices segment also holds significant importance, with Sleep Test Devices capturing a substantial portion due to the growing awareness and diagnosis of sleep disorders. These devices contribute approximately 25% to the diagnostic segment's market share.
Key drivers contributing to the dominance of therapeutic devices, particularly PAP devices, include:
- Rising Prevalence of Sleep Apnea: A significant portion of the Italian population suffers from OSA, creating a consistent demand for PAP therapy.
- Technological Advancements in PAP Devices: Innovations like humidification control, comfortable mask designs, and real-time data reporting enhance patient compliance and therapeutic efficacy.
- Government Initiatives and Reimbursement Policies: Favorable reimbursement schemes for sleep apnea treatment in Italy encourage the adoption of PAP devices.
- Increased Physician Awareness and Prescriptions: Healthcare professionals are increasingly recognizing the importance of PAP therapy for OSA management.
The Disposables segment, while supporting the overall market, is largely dependent on the sales of the primary therapeutic and diagnostic devices.
The dominance is further solidified by economic policies that support the adoption of advanced medical technologies and healthcare infrastructure that facilitates home-based patient care. The growth potential within this segment is substantial, projected to continue its upward trajectory as awareness and treatment options expand. Market share within the therapeutic devices segment for PAP devices is estimated at 35%, with a projected growth rate of 13% over the forecast period.
Italy Digital Respiratory Devices Market Product Landscape
The product landscape of the Italy digital respiratory devices market is characterized by innovative solutions designed to enhance patient outcomes and streamline healthcare delivery. Key product categories include advanced spirometers with cloud connectivity for effortless data sharing and remote analysis, sophisticated sleep test devices that offer both in-lab and at-home diagnostics with enhanced comfort and accuracy, and a range of therapeutic devices such as intelligent Positive Airway Pressure (PAP) devices featuring personalized therapy settings and seamless integration with mobile apps for patient tracking. Furthermore, innovative nebulizers and inhalers are incorporating digital features for dose tracking and adherence monitoring, while specialized humidifiers are designed for optimal patient comfort. The emphasis is on user-friendly interfaces, robust data analytics capabilities, and miniaturized form factors for increased portability and patient comfort. The unique selling propositions often lie in the accuracy of data capture, the ease of integration with existing healthcare systems, and the ability to provide actionable insights for both patients and clinicians. Technological advancements are continuously pushing the boundaries, with a focus on AI-powered diagnostics and predictive capabilities.
Key Drivers, Barriers & Challenges in Italy Digital Respiratory Devices Market
The Italy digital respiratory devices market is propelled by several key drivers, including the increasing prevalence of chronic respiratory diseases such as COPD and asthma, a growing aging population with a higher susceptibility to respiratory ailments, and the accelerating adoption of telehealth and remote patient monitoring solutions. Technological advancements in IoT, AI, and sensor technology are enabling the development of more sophisticated and user-friendly digital respiratory devices. Government initiatives promoting digital health and increasing healthcare expenditure further support market growth.
However, the market also faces significant barriers and challenges. These include the high cost of advanced digital respiratory devices, which can limit accessibility for some patient segments, and the need for robust cybersecurity measures to protect sensitive patient data. Regulatory hurdles and the complexities of obtaining reimbursement for new digital health technologies can also impede market expansion. Furthermore, a lack of widespread digital literacy among certain patient demographics and healthcare professionals can hinder adoption rates. Supply chain disruptions and the dependency on global component sourcing present potential risks, while intense competition from both established medical device companies and emerging tech startups requires continuous innovation and strategic positioning.
Emerging Opportunities in Italy Digital Respiratory Devices Market
Emerging opportunities in the Italy digital respiratory devices market lie in the expansion of personalized respiratory care through AI-driven diagnostics and treatment optimization. The untapped potential of the pediatric respiratory segment, with the development of child-friendly digital monitoring tools, presents a significant avenue for growth. Furthermore, the integration of digital respiratory devices with broader digital health ecosystems and wearable health trackers offers synergistic opportunities for comprehensive health management. The growing demand for subscription-based service models for digital respiratory devices, encompassing ongoing support and data analysis, is another promising area. The development of advanced predictive analytics for early detection of exacerbations in chronic respiratory diseases also represents a key opportunity to improve patient outcomes and reduce healthcare costs.
Growth Accelerators in the Italy Digital Respiratory Devices Market Industry
The long-term growth of the Italy digital respiratory devices market is being significantly accelerated by ongoing technological breakthroughs in areas such as miniaturized biosensors, advanced AI algorithms for real-time data interpretation, and the development of more efficient and secure wireless communication technologies. Strategic partnerships between medical device manufacturers, technology companies, and healthcare providers are crucial for fostering innovation and ensuring seamless integration of digital solutions into clinical workflows. Market expansion strategies, including increased focus on direct-to-consumer sales channels and the development of user-friendly mobile applications, are also playing a vital role in driving adoption. The increasing recognition by healthcare systems of the cost-effectiveness and improved patient outcomes associated with remote monitoring and digital interventions will continue to be a major catalyst for sustained growth in this dynamic market.
Key Players Shaping the Italy Digital Respiratory Devices Market Market
- Medtronic PLC
- Fosun Pharmaceutical (Breas Medical AB)
- Koninklijke Philips NV
- Invacare Corporation
- ICU Medical (Smiths Medical Inc )
- GE Healthcare
- Dragerwerk AG
- Fisher & Paykel Healthcare Ltd
- GlaxoSmithKline PLC
Notable Milestones in Italy Digital Respiratory Devices Market Sector
- July 2022: The Bastide Group announced the purchase of a majority stake in Oxystore, an Italian company near Milan. Oxystore specializes in consumer home diagnosis and online sales of oxygen therapy and sleep apnea devices, also providing support and follow-up services to patients receiving home treatment.
- February 2022: ProSomnus Sleep Technologies, a leader in patient-preferred medical devices for the treatment of obstructive sleep apnea (OSA), displayed four poster abstracts of its oral appliance therapy (OAT) devices at the World Sleep Congress 2022, which was held in March 2022, in Rome, Italy.
In-Depth Italy Digital Respiratory Devices Market Market Outlook
- July 2022: The Bastide Group announced the purchase of a majority stake in Oxystore, an Italian company near Milan. Oxystore specializes in consumer home diagnosis and online sales of oxygen therapy and sleep apnea devices, also providing support and follow-up services to patients receiving home treatment.
- February 2022: ProSomnus Sleep Technologies, a leader in patient-preferred medical devices for the treatment of obstructive sleep apnea (OSA), displayed four poster abstracts of its oral appliance therapy (OAT) devices at the World Sleep Congress 2022, which was held in March 2022, in Rome, Italy.
In-Depth Italy Digital Respiratory Devices Market Market Outlook
The Italy digital respiratory devices market is poised for substantial expansion, driven by the convergence of technological innovation, a growing healthcare burden from respiratory conditions, and a proactive approach towards digital transformation in healthcare. Future market potential is significantly enhanced by the increasing demand for personalized medicine and home-based care solutions. Strategic opportunities lie in the development of integrated platforms that combine diagnostic, therapeutic, and predictive capabilities, along with robust data analytics for improved patient management and research. The market is expected to witness further consolidation and strategic alliances as companies seek to leverage synergistic strengths and expand their global reach within the European digital health market. Addressing evolving regulatory landscapes and ensuring equitable access to advanced digital respiratory technologies will be crucial for realizing the full growth potential and improving respiratory health outcomes across Italy.
Italy Digital Respiratory Devices Market Segmentation
-
1. Type
-
1.1. By Diagnostic and Monitoring Devices
- 1.1.1. Spirometers
- 1.1.2. Sleep Test Devices
- 1.1.3. Other Diagnostic and Monitoring Devices
-
1.2. By Therapeutic Devices
- 1.2.1. Positive Airway Pressure (PAP) Devices
- 1.2.2. Humidifiers
- 1.2.3. Nebulizers
- 1.2.4. Ventilators
- 1.2.5. Inhalers
- 1.2.6. Other Therapeutic Devices
- 1.3. By Disposables
-
1.1. By Diagnostic and Monitoring Devices
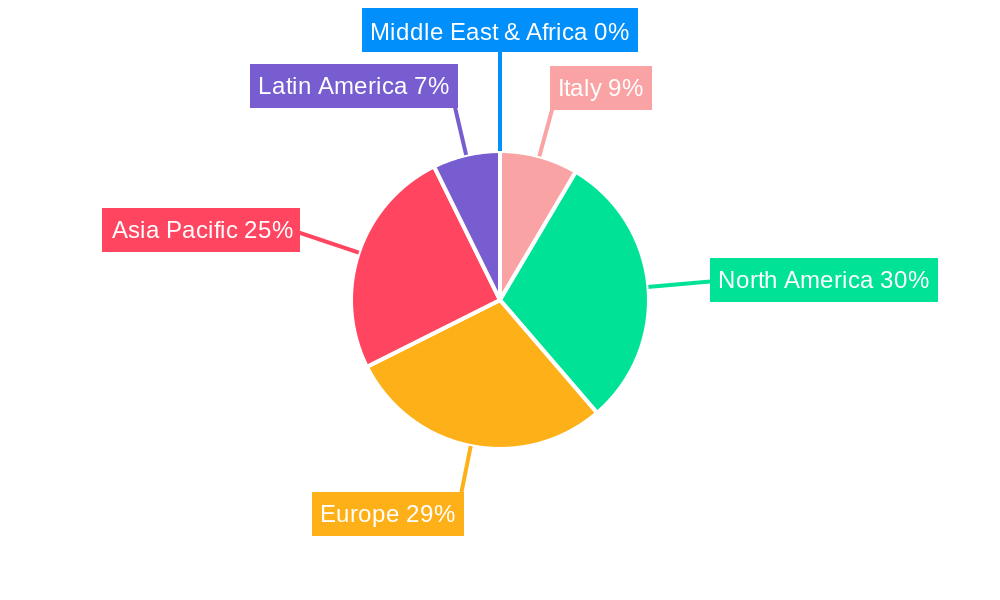
Italy Digital Respiratory Devices Market Segmentation By Geography
- 1. Italy
Italy Digital Respiratory Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.80% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Respiratory Disorders; Technological Advancements and Increasing Applications in Homecare Settings
- 3.3. Market Restrains
- 3.3.1. High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Ventilators as Therapeutic Devices Expected to Register Significant Growth
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Italy Digital Respiratory Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. By Diagnostic and Monitoring Devices
- 5.1.1.1. Spirometers
- 5.1.1.2. Sleep Test Devices
- 5.1.1.3. Other Diagnostic and Monitoring Devices
- 5.1.2. By Therapeutic Devices
- 5.1.2.1. Positive Airway Pressure (PAP) Devices
- 5.1.2.2. Humidifiers
- 5.1.2.3. Nebulizers
- 5.1.2.4. Ventilators
- 5.1.2.5. Inhalers
- 5.1.2.6. Other Therapeutic Devices
- 5.1.3. By Disposables
- 5.1.1. By Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Italy
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2024
- 6.2. Company Profiles
- 6.2.1 Medtronic PLC
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Fosun Pharmaceutical (Breas Medical AB)
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Koninklijke Philips NV
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Invacare Corporation
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 ICU Medical (Smiths Medical Inc )
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 GE Healthcare
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Dragerwerk AG
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Fisher & Paykel Healthcare Ltd
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 GlaxoSmithKline PLC
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.1 Medtronic PLC
List of Figures
- Figure 1: Italy Digital Respiratory Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Italy Digital Respiratory Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 4: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Type 2019 & 2032
- Table 5: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 7: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 9: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 10: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Type 2019 & 2032
- Table 11: Italy Digital Respiratory Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Italy Digital Respiratory Devices Market Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Italy Digital Respiratory Devices Market?
The projected CAGR is approximately 6.80%.
2. Which companies are prominent players in the Italy Digital Respiratory Devices Market?
Key companies in the market include Medtronic PLC, Fosun Pharmaceutical (Breas Medical AB), Koninklijke Philips NV, Invacare Corporation, ICU Medical (Smiths Medical Inc ), GE Healthcare, Dragerwerk AG, Fisher & Paykel Healthcare Ltd, GlaxoSmithKline PLC.
3. What are the main segments of the Italy Digital Respiratory Devices Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders; Technological Advancements and Increasing Applications in Homecare Settings.
6. What are the notable trends driving market growth?
Ventilators as Therapeutic Devices Expected to Register Significant Growth.
7. Are there any restraints impacting market growth?
High Cost of Devices.
8. Can you provide examples of recent developments in the market?
In July 2022, the Bastide Group announced the purchase of a majority stake in Oxystore, an Italian company near Milan. Oxystore specializes in consumer home diagnosis and online sales of oxygen therapy and sleep apnea devices. It also provides support and follow-up services to patients receiving home treatment.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Italy Digital Respiratory Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Italy Digital Respiratory Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Italy Digital Respiratory Devices Market?
To stay informed about further developments, trends, and reports in the Italy Digital Respiratory Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



