Key Insights
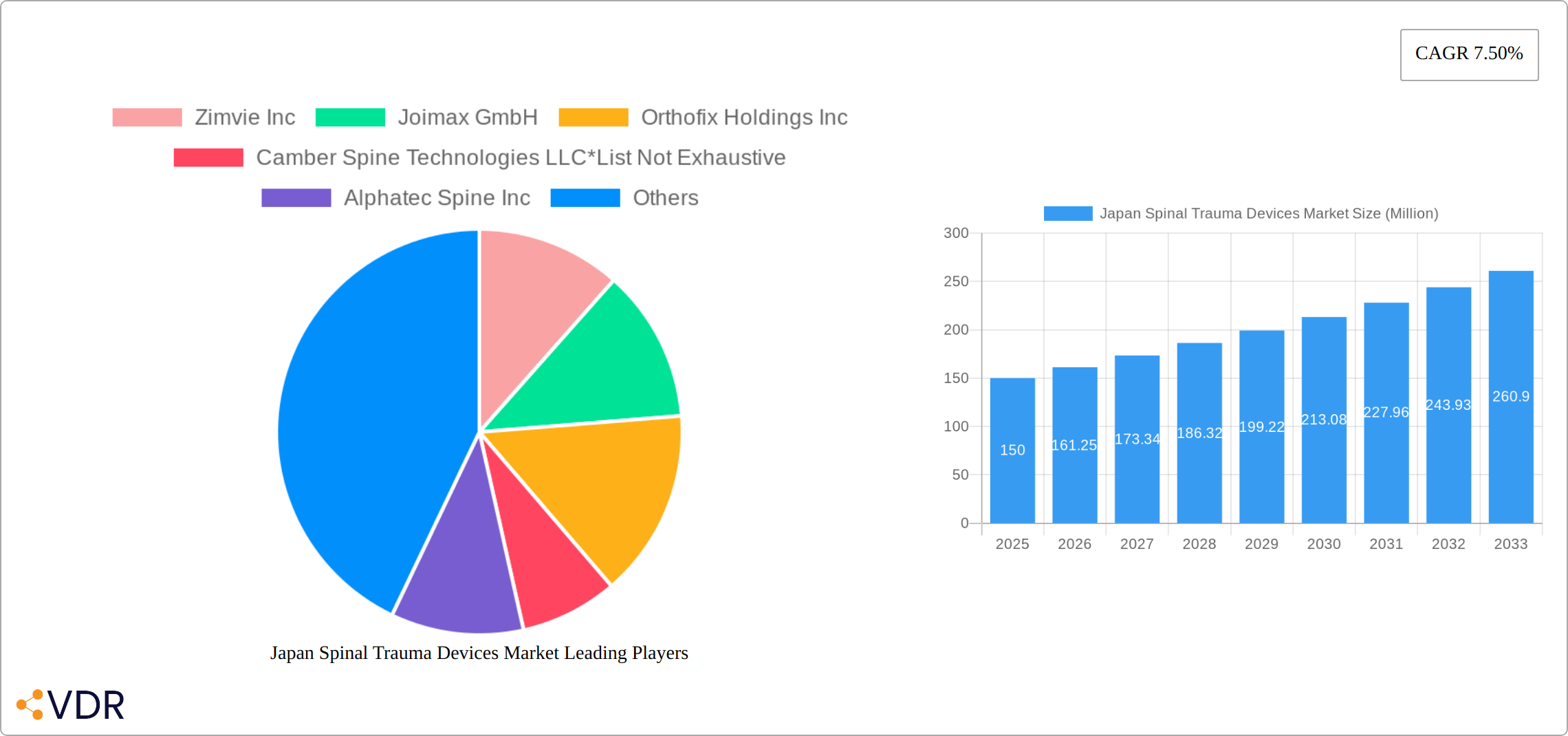
The Japan spinal trauma devices market, valued at approximately ¥150 million (estimated based on common market sizes for similar regions and the provided CAGR) in 2025, is projected to experience robust growth with a Compound Annual Growth Rate (CAGR) of 7.50% from 2025 to 2033. This expansion is driven by several key factors. An aging population in Japan is leading to a higher incidence of osteoporosis and related spinal fractures, fueling demand for effective trauma devices. Furthermore, advancements in minimally invasive surgical techniques and the development of innovative, biocompatible materials are contributing to improved treatment outcomes and increased market adoption. The rising prevalence of road accidents and other trauma incidents also adds to the market's growth momentum. However, high healthcare costs and stringent regulatory approvals in Japan pose challenges to market expansion. The market is segmented by device type (spinal decompression, spinal fusion, fracture repair, and others) and geographically across regions like Kanto, Kansai, Chubu, Kyushu, and Tohoku, each exhibiting varying growth rates based on population density and healthcare infrastructure.
The competitive landscape is characterized by the presence of both global and domestic players, including prominent names such as Medtronic PLC, Stryker Corporation, NuVasive Inc, and others. These companies are engaged in strategic initiatives like product innovation, partnerships, and acquisitions to strengthen their market positions. The continued focus on technological advancements, including the development of smart implants and personalized medicine approaches, will further shape the market trajectory. The growth trajectory is expected to be influenced by government initiatives to improve healthcare access and outcomes, alongside the evolving preferences of surgeons and patients toward minimally invasive procedures and advanced implant technologies. Within the segmentation, spinal fusion devices are expected to hold a significant market share, given their established role in addressing complex spinal trauma cases. The forecast period underscores a promising outlook for the Japan spinal trauma devices market, promising substantial opportunities for market participants and signifying a positive outlook for patient care.
Japan Spinal Trauma Devices Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Japan Spinal Trauma Devices Market, encompassing market dynamics, growth trends, regional performance, product landscape, key players, and future outlook. The study period covers 2019-2033, with 2025 as the base and estimated year. The report is invaluable for industry professionals, investors, and strategic decision-makers seeking actionable insights into this evolving market. The total market size is projected to reach xx Million units by 2033.
Japan Spinal Trauma Devices Market Market Dynamics & Structure
The Japan spinal trauma devices market is characterized by a moderately concentrated landscape, with key players like Medtronic PLC, Stryker Corporation, NuVasive Inc, Globus Medical Inc, Abbott Laboratories, and Otsuka Medical holding significant market share. However, the market also features several smaller, specialized companies like Zimvie Inc, Joimax GmbH, Orthofix Holdings Inc, and Camber Spine Technologies LLC, contributing to a dynamic competitive environment. The market structure is influenced by several factors:
- Technological Innovation: The adoption of advanced technologies like 3D printing (as seen with SeaSpine's WaveForm TO system) and augmented reality surgical navigation (Philips' ClarifEye) is driving significant growth. However, high initial investment costs and the need for specialized training pose innovation barriers.
- Regulatory Framework: Stringent regulatory approvals in Japan influence product launch timelines and market entry strategies. Compliance with Japanese regulatory standards is crucial for market success.
- Competitive Product Substitutes: The availability of alternative treatment methods and devices creates competitive pressure, necessitating continuous product improvement and innovation.
- End-User Demographics: The aging population in Japan contributes significantly to the demand for spinal trauma devices. The increasing prevalence of age-related spinal disorders fuels market growth.
- M&A Trends: The market has witnessed a moderate level of mergers and acquisitions in recent years, with xx major deals recorded between 2019 and 2024, contributing to market consolidation and expansion of product portfolios. This trend is expected to continue, driving further market concentration. The average deal size was approximately xx Million units.
Japan Spinal Trauma Devices Market Growth Trends & Insights
The Japan spinal trauma devices market exhibited a CAGR of xx% during the historical period (2019-2024), reaching xx Million units in 2024. This growth is primarily attributed to the increasing prevalence of spinal trauma and disorders, the rising geriatric population, and technological advancements leading to improved treatment outcomes. The market is expected to maintain a robust growth trajectory during the forecast period (2025-2033), projected to reach xx Million units by 2033, with a CAGR of xx%. This growth will be driven by factors such as:
- Rising incidence of spinal trauma and degenerative diseases: The aging population and increasing accident rates contribute to a greater demand for spinal trauma devices.
- Technological advancements: Innovation in minimally invasive surgical techniques and advanced materials enhance treatment efficacy and patient outcomes.
- Growing awareness and improved healthcare infrastructure: Increased patient awareness and access to better healthcare facilities contribute to the adoption of advanced spinal trauma devices.
- Government initiatives and insurance coverage: Government support for healthcare infrastructure and insurance coverage for spinal trauma treatment contribute to market growth.
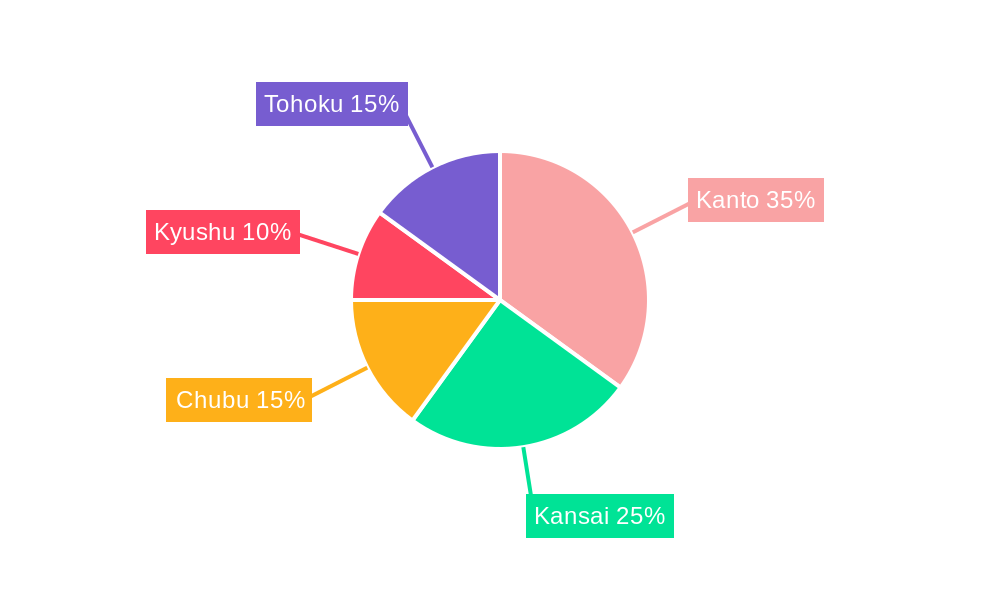
Dominant Regions, Countries, or Segments in Japan Spinal Trauma Devices Market
The Kanto region, encompassing Tokyo and surrounding prefectures, holds a significant share of the Japan spinal trauma devices market in 2024. While precise market share percentages are proprietary information, its dominance is attributable to several key factors:
- High Concentration of Medical Facilities and Skilled Professionals: The Kanto region boasts a concentration of leading hospitals and medical centers equipped with advanced surgical technologies and staffed by highly experienced spinal surgeons. This concentration creates a higher demand for sophisticated spinal trauma devices.
- Large Patient Population: The region's substantial population density translates into a larger pool of patients requiring spinal trauma interventions, directly driving demand.
- Advanced Healthcare Infrastructure and Reimbursement Systems: The region's superior healthcare infrastructure, including robust reimbursement systems, facilitates the adoption and accessibility of advanced spinal trauma devices, accelerating market penetration.
Analyzing the product segments, Spinal Fusion devices maintain the largest market share, followed by Fracture Repair and Spinal Decompression devices. The "Others" category, encompassing specialized devices and emerging technologies, represents a smaller yet dynamically growing segment. This market segmentation reflects the high prevalence of conditions necessitating spinal fusion and fracture repair procedures in Japan.
Japan Spinal Trauma Devices Market Product Landscape
The Japan spinal trauma devices market showcases a diverse product landscape, encompassing a wide array of implants, instruments, and related accessories. Recent innovations include 3D-printed interbody systems offering improved biocompatibility and customized fit, as well as augmented reality surgical navigation systems enhancing precision and minimally invasive procedures. These advancements offer improved surgical outcomes, reduced recovery times, and enhanced patient satisfaction, driving market growth.
Key Drivers, Barriers & Challenges in Japan Spinal Trauma Devices Market
Key Drivers:
- Technological advancements: Continuous innovation in materials, designs, and surgical techniques improve treatment outcomes and drive adoption.
- Aging population: The increasing prevalence of age-related spinal disorders fuels market demand.
- Rising healthcare expenditure: Increased investment in healthcare infrastructure and technology boosts market growth.
Key Challenges:
- High cost of devices: The high price of advanced spinal trauma devices limits accessibility for some patients.
- Stringent regulatory approvals: The lengthy regulatory process for new product launches can create delays.
- Competition from established players: The presence of major international players creates intense competition, impacting market share for smaller companies. This competition resulted in a price war reducing average revenue by xx% in 2024 compared to 2023.
Emerging Opportunities in Japan Spinal Trauma Devices Market
Emerging opportunities include the increasing adoption of minimally invasive surgical techniques, the growing demand for personalized medicine, and the potential for integrating AI and machine learning into surgical planning and execution. Untapped markets include regional areas outside the major urban centers, where healthcare infrastructure is still developing.
Growth Accelerators in the Japan Spinal Trauma Devices Market Industry
Strategic partnerships between device manufacturers and hospitals or research institutions are accelerating market growth. Furthermore, government initiatives promoting the adoption of innovative medical technologies create a favorable environment. Continuous investment in research and development of new devices with improved functionalities will further propel market expansion.
Key Players Shaping the Japan Spinal Trauma Devices Market Market
- Zimvie Inc
- Joimax GmbH
- Orthofix Holdings Inc
- Camber Spine Technologies LLC
- Alphatec Spine Inc
- Otsuka Medical
- Abbott Laboratories
- Globus Medical Inc
- Medtronic PLC
- B Braun Melsungen AG
- Stryker Corporation
- NuVasive Inc
Notable Milestones in Japan Spinal Trauma Devices Market Sector
- October 2022: Philips launched ClarifEye AR surgical navigation solution in Japan, achieving positive results in Tokyo.
- June 2022: SeaSpine Holdings Corporation launched its 3D-printed WaveForm TO Interbody System.
In-Depth Japan Spinal Trauma Devices Market Market Outlook
The Japan spinal trauma devices market is poised for continued growth, driven by technological advancements, an aging population, and expanding healthcare infrastructure. Strategic investments in research and development, coupled with strategic partnerships, will further accelerate market expansion, creating lucrative opportunities for both established players and emerging companies. The market is expected to witness strong growth in the coming years, with significant potential for innovation and market consolidation.
Japan Spinal Trauma Devices Market Segmentation
-
1. Type
-
1.1. Spinal Decompression
- 1.1.1. Corpectomy
- 1.1.2. Discectomy
- 1.1.3. Laminotomy
- 1.1.4. Others
- 1.2. Spinal Fusion
- 1.3. Fracture Repair
-
1.1. Spinal Decompression
Japan Spinal Trauma Devices Market Segmentation By Geography
- 1. Japan
Japan Spinal Trauma Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 7.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Adoption Rate of Minimally Invasive Spinal Surgeries; Increasing Incidences of Obesity and Degenerative Spinal Conditions; Rising Geriatric Popualtion in Japan
- 3.3. Market Restrains
- 3.3.1. Risks Associated with Spinal Surgeries; High Cost and Time Involved in Treatment Procedures
- 3.4. Market Trends
- 3.4.1. Spinal Fusion Surgery Devices Segment is Expected to Cover a Large Share of the Market Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Spinal Decompression
- 5.1.1.1. Corpectomy
- 5.1.1.2. Discectomy
- 5.1.1.3. Laminotomy
- 5.1.1.4. Others
- 5.1.2. Spinal Fusion
- 5.1.3. Fracture Repair
- 5.1.1. Spinal Decompression
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Japan
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Kanto Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 7. Kansai Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 8. Chubu Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 9. Kyushu Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 10. Tohoku Japan Spinal Trauma Devices Market Analysis, Insights and Forecast, 2019-2031
- 11. Competitive Analysis
- 11.1. Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Zimvie Inc
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Joimax GmbH
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Orthofix Holdings Inc
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Camber Spine Technologies LLC*List Not Exhaustive
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Alphatec Spine Inc
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Otsuka Medical
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Abbott Laboratories
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Globus Medical Inc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Medtronic PLC
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 B Braun Melsungen AG
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Stryker Corporation
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 NuVasive Inc
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.1 Zimvie Inc
List of Figures
- Figure 1: Japan Spinal Trauma Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Japan Spinal Trauma Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 3: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 4: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 5: Kanto Japan Spinal Trauma Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 6: Kansai Japan Spinal Trauma Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Chubu Japan Spinal Trauma Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Kyushu Japan Spinal Trauma Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Tohoku Japan Spinal Trauma Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Type 2019 & 2032
- Table 11: Japan Spinal Trauma Devices Market Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Japan Spinal Trauma Devices Market?
The projected CAGR is approximately 7.50%.
2. Which companies are prominent players in the Japan Spinal Trauma Devices Market?
Key companies in the market include Zimvie Inc, Joimax GmbH, Orthofix Holdings Inc, Camber Spine Technologies LLC*List Not Exhaustive, Alphatec Spine Inc, Otsuka Medical, Abbott Laboratories, Globus Medical Inc, Medtronic PLC, B Braun Melsungen AG, Stryker Corporation, NuVasive Inc.
3. What are the main segments of the Japan Spinal Trauma Devices Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Adoption Rate of Minimally Invasive Spinal Surgeries; Increasing Incidences of Obesity and Degenerative Spinal Conditions; Rising Geriatric Popualtion in Japan.
6. What are the notable trends driving market growth?
Spinal Fusion Surgery Devices Segment is Expected to Cover a Large Share of the Market Over the Forecast Period.
7. Are there any restraints impacting market growth?
Risks Associated with Spinal Surgeries; High Cost and Time Involved in Treatment Procedures.
8. Can you provide examples of recent developments in the market?
October 2022: Philips expanded the rollout of its augmented reality (AR) surgical navigation solution, ClrifEye, to Japan, highlighting positive results in the first patients treated in Tokyo, Japan. ClarifEue helped the hospital's surgeons to successfully treat patients with spinal stenosis and scoliosis via minimally-invasive image-guided procedures.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Japan Spinal Trauma Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Japan Spinal Trauma Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Japan Spinal Trauma Devices Market?
To stay informed about further developments, trends, and reports in the Japan Spinal Trauma Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



